18106
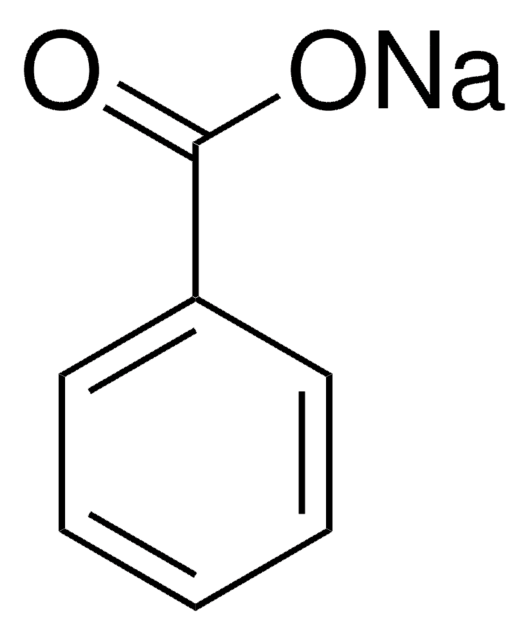
Sodium benzoate
puriss., meets analytical specification of Ph. Eur., BP, FCC, E211, 99.0-100.5% (calc. to the dried substance), powder
Synonym(s):
BENZOTRON™, Benzoic acid sodium salt
About This Item
Recommended Products
grade
puriss.
Quality Level
assay
99.0-100.5% (calc. to the dried substance)
form
powder
quality
meets analytical specification of Ph. Eur., BP, FCC, E211
impurities
acidity or alkalinity, complies
oxidizable impurities, complies
residual solvents, complies
≤0.001% heavy metals (as Pb)
≤0.03% halogenated compounds (as Cl)
loss
≤1% loss on drying, 105 °C
mp
>300 °C (lit.)
solubility
water: soluble 556 g/L
anion traces
chloride (Cl-): ≤100 mg/kg
sulfate (SO42-): ≤100 mg/kg
cation traces
As: ≤2 mg/kg
Cu: ≤10 mg/kg
Hg: ≤1 mg/kg
Pb: ≤2 mg/kg
Zn: ≤10 mg/kg
suitability
complies for appearance of solution
SMILES string
[Na+].[O-]C(=O)c1ccccc1
InChI
1S/C7H6O2.Na/c8-7(9)6-4-2-1-3-5-6;/h1-5H,(H,8,9);/q;+1/p-1
InChI key
WXMKPNITSTVMEF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- 2-Phenoxyethyl benzoate via phase-transfercatalyzed esterification with 2-phenoxyethyl bromide.
- Ynones by Pd free coupling reactionwith terminal alkynes in the presence of cyanuric chloride and magnesiumchloride.
- 3-Methyl-4-arylmethylene-isoxazol-5(4H)-onesby reacting ethyl acetoacetate, hydroxylamine hydrochloride, and aromaticaldehydes.
- 4-Pyrazolylmethylene-3-phenylisoxazol-5(4H)-onesvia three-component cyclocondensation of pyrazole-4-carbaxaldehyde withβ-ketoesters and hydroxylamine hydrochloride.
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service