251275
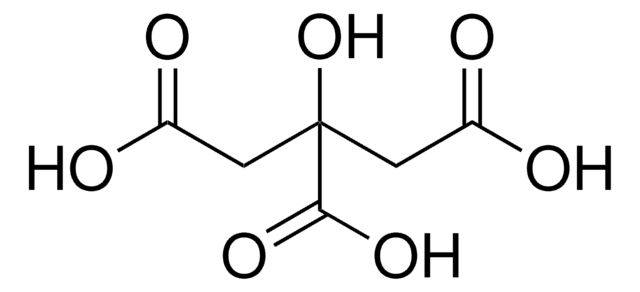
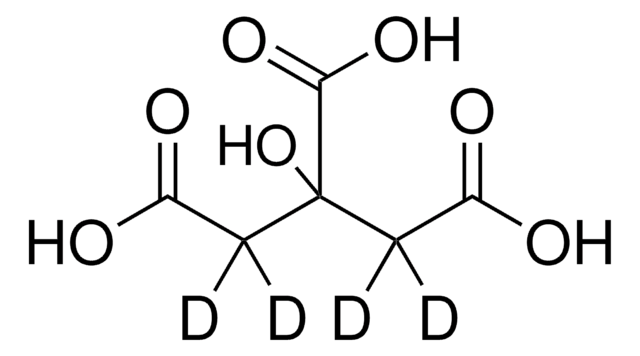
Citric acid
ACS reagent, ≥99.5%
Synonym(s):
2-Hydroxy-1,2,3-propanetricarboxylic acid, 2-Hydroxypropan-1,2,3-tricarboxylic acid
About This Item
Recommended Products
grade
ACS reagent
Quality Level
assay
≥99.5%
form
crystals
expl. lim.
8 %, 65 °F
impurities
Substances carbonizable by hot sulfuric acid, passes test
ign. residue
≤0.02%
pKa
(1) 3.13, (2) 4.76, (3) 6.4
mp
153-159 °C (lit.)
anion traces
chloride (Cl-): ≤0.001%
oxalate (C2O42-): passes test (limit about 0.003%)
phosphate (PO43-): ≤0.001%
sulfate (SO42-): ≤0.002%
cation traces
Fe: ≤3 ppm
Pb: ≤2 ppm
SMILES string
OC(=O)CC(O)(CC(O)=O)C(O)=O
InChI
1S/C6H8O7/c7-3(8)1-6(13,5(11)12)2-4(9)10/h13H,1-2H2,(H,7,8)(H,9,10)(H,11,12)
InChI key
KRKNYBCHXYNGOX-UHFFFAOYSA-N
Gene Information
human ... SRC(6714)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Phosphate citrate buffer for use in enzyme-linked immunosorbent assay.
- Citrate-stabilized ceria aqueous sol, which was employed in the synthesis of cerium oxide nanoparticles.
- Citric acid-Na2HPO4-buffered stock solution for use in the determination of fecal urease activity.
- Anticoagulant citrate dextrose solution A (ACD-A), which is employed during the isolation of blood-derived endothelial progenitor cells.
Citric acid has also been used:
- In a novel process which allows controlling of the particle size during the synthesis of palladium cuboctahedrons.
- To prepare citric acid-derived carbon nanodots (CNDs) by bottom-up carbonization method.
- As a bi-component chelating agent for the synthesis of Li4Ti5O12 (lithium titanate oxide) by a novel sol–gel method.
Other Notes
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
A complete workflow for the intact and middle-up mass analysis of reduced and non-reduced monoclonal antibodies based on SEC-MS with sample preparation by protein-A affinity clean-up.
Related Content
Step-by-step reversed phase UHPLC-MS workflow for middle-up mass analysis of an immunoglobulin G antibody, consisting of antibody purification, IdeS proteolysis and reduction, mass spectrometer calibration, mAb quantification, and a system suitability test.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service