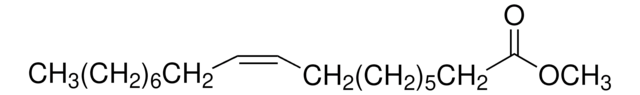
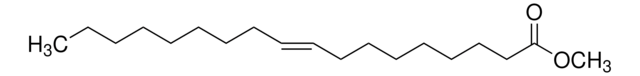
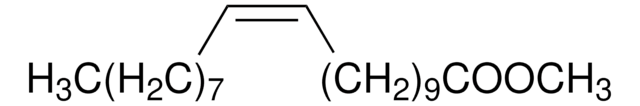
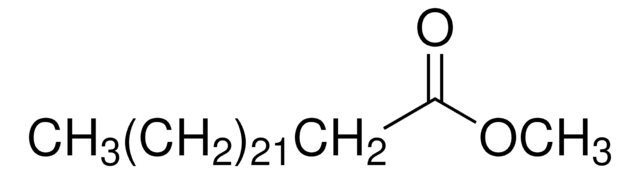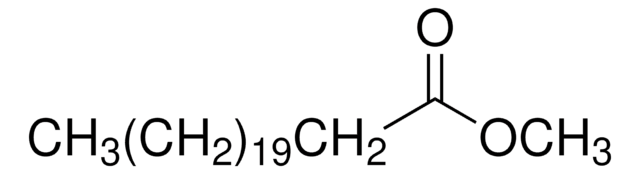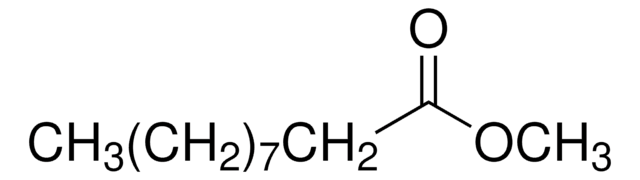75160
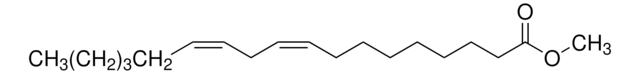
Methyl oleate
analytical standard
Synonym(s):
Methyl cis-9-octadecenoate, Oleic acid methyl ester
About This Item
Recommended Products
grade
analytical standard
Quality Level
vapor pressure
10 mmHg ( 205 °C)
assay
≥98.5% (GC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
refractive index
n20/D 1.452 (lit.)
n20/D 1.452
bp
218 °C/20 mmHg (lit.)
density
0.874 g/mL at 20 °C (lit.)
application(s)
cleaning products
cosmetics
flavors and fragrances
food and beverages
personal care
format
neat
functional group
ester
shipped in
ambient
storage temp.
2-8°C
SMILES string
CCCCCCCC\C=C/CCCCCCCC(=O)OC
InChI
1S/C19H36O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19(20)21-2/h10-11H,3-9,12-18H2,1-2H3/b11-10-
InChI key
QYDYPVFESGNLHU-KHPPLWFESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Determination of free fatty acids in plasma samples following their methylation by gas chromatography (GC)
- Comparative analysis of gas chromatography-combustion-mass spectrometry and gas chromatography-flame ionization detector methods for the determination of fatty acid methyl esters (FAMEs) in biodiesel samples
- Simultaneous determination of fatty acid methyl esters in commercial food oil samples by gas chromatography-vacuum ultraviolet (GC-VUV) spectroscopy
- Measurement of fatty acid methyl ester composition of various edible oil samples by 1H nuclear magnetic resonance (1H NMR) spectroscopy combined with partial least squares (PLS) method
- Simultaneous determination of fatty acids in bovine colostrum samples by GC-FID after their derivatization to ester forms using an acidic catalyst boron trifluoride
Other Notes
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
235.4 °F - closed cup
flash_point_c
113.0 °C - closed cup
ppe
Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Separation of Methyl oleate; Caprylic acid; Heptanoic acid; Methyl decanoate; Methyl dodecanoate; Myristic acid; Methyl palmitate; Methyl palmitoleate; Methyl stearate; Methyl linoleate; Methyl linolenate; Acetic acid; Arachidic acid; Behenic acid; Propionic acid; Isobutyric acid; Valeric acid; Isovaleric acid; Isocaproic acid; Butyric acid
Protocols
Separation of Methyl erucate; Methyl palmitate; Methyl stearate; Methyl linolenate; Methyl eicosenoate; Methyl behenate; Methyl myristate; Methyl oleate; Methyl arachidate
Separation of Methyl decanoate; Methyl dodecanoate; Methyl myristate; Methyl palmitate; Methyl caprylate; Methyl oleate; Methyl linoleate; Methyl linolenate; Methyl stearate
-11-eicosenoate; Methyl elaidate; Methyl linoleate; Methyl myristate; Methyl myristoleate; Methyl palmitate; Methyl palmitoleate; Methyl oleate; Methyl pentadecanoate; Methyl tridecanoate; Methyl behenate; Methyl caprylate; Methyl erucate; Methyl heptadecanoate; Methyl arachidate
GC Analysis of a 37-Component FAME Mix on Omegawax® (15 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service