87920
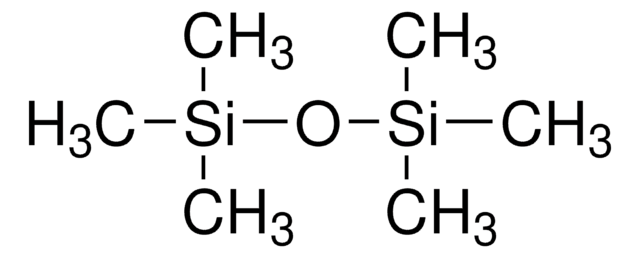
Tetramethylsilane
analytical standard, for NMR spectroscopy, ACS reagent
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
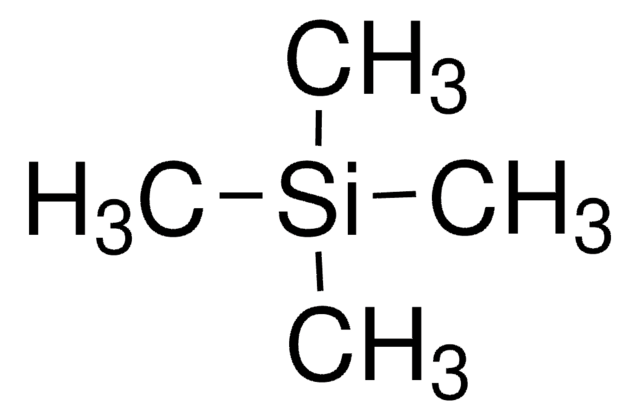
Linear Formula:
Si(CH3)4
CAS Number:
Molecular Weight:
88.22
Beilstein/REAXYS Number:
1696908
EC Number:
MDL number:
UNSPSC Code:
12142201
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
ACS reagent
analytical standard
for NMR spectroscopy
Quality Level
vapor pressure
11.66 psi ( 20 °C)
assay
≥99.5% (GC)
form
liquid
autoignition temp.
842 °F
refractive index
n20/D 1.358 (lit.)
n20/D 1.359
bp
26-28 °C (lit.)
mp
−99 °C (lit.)
density
0.648 g/mL at 25 °C (lit.)
application(s)
environmental
format
neat
storage temp.
2-8°C
SMILES string
C[Si](C)(C)C
InChI
1S/C4H12Si/c1-5(2,3)4/h1-4H3
InChI key
CZDYPVPMEAXLPK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Tetramethylsilane has been recommended by the International Union of Pure and Applied Chemistry (IUPAC) as a universal reference for all nuclides. It is commonly used as a reference standard in nuclear magnetic resonance (NMR) for measuring proton chemical shifts and the temperature dependence of the 1H chemical shift of TMS in solvents such as chloroform, methanol, and dimethylsulfoxide is studied.
Application
Tetramethylsilane may be used as an internal standard for the quantitative analysis of medicinal plant extracts and herbal products using the quantitative NMR (qNMR) method. It may also be used as an internal standard to investigate peroxide-based chemical systems for the crosslinking reactions carried on isotactic polypropylene using FT-IR spectrometry and proton nuclear magnetic resonance (1H NMR) techniques.
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ramesh C Sharma et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 65(3-4), 787-791 (2006-03-15)
The decomposition of trimethylsilane and tetramethylsilane has been investigated for the first time, using hot wire (catalytic) at various temperatures. Trimethylsilane is catalytic-dissociated in these species SiH(2), CH(3)SiH, CH(3), CH(2)Si. Time of flight mass spectroscopy signal of these species are
K Eberle et al.
Physics in medicine and biology, 48(21), 3555-3564 (2003-12-05)
First measurements with a prototype ionization chamber are described to be applied in online monitoring of modulated fields in radiation therapy. The liquids isooctane, isononane (TMP) and tetramethylsilane (TMS) are used in a high purity grade in order to realize
Robin K Harris et al.
Magnetic resonance in chemistry : MRC, 45 Suppl 1, S174-S186 (2007-12-25)
Computations for chemical shifts of molecular organic compounds using the gauge-including projector augmented wave method and the NMR-CASTEP code are reviewed. The methods are briefly introduced, and some general aspects involving the sources of uncertainty in the results are explored.
Roy E Hoffman
Journal of magnetic resonance (San Diego, Calif. : 1997), 163(2), 325-331 (2003-08-14)
The chemical shift of TMS is commonly assumed to be zero. However, it varies by over 1 ppm for 1H and 4 ppm for 13C and shows a correlation with the physical properties of the solvent. Using the commonly accepted
Maria T Proetto et al.
ChemMedChem, 9(6), 1176-1187 (2014-05-23)
A series of methoxy- and fluorine-substituted [salophene]platinum(II) complexes (salophene=N,N'-bis(salicylidene)-1,2-phenylenediamine) were synthesized and characterized by (1) H NMR spectroscopy and mass spectrometry. The structure was confirmed on the example of [5-OCH3 -salophene]platinum(II) (4-Pt) by crystal structure analysis. The cytotoxicity of all
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service