90520
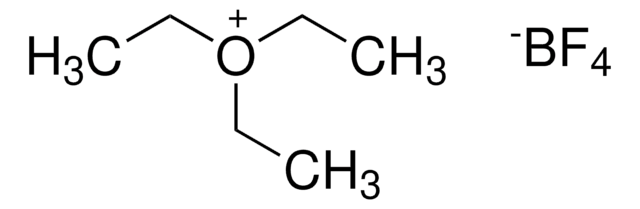
Triethyloxonium tetrafluoroborate
≥97.0% (T)
Synonym(s):
Et3OBF4, Meerwein′s reagent, Triethyloxonium fluoroborate
About This Item
Recommended Products
Quality Level
assay
≥97.0% (T)
form
crystals
contains
1-3% ether as stabilizer
solubility
methylene chloride: 20 mg/mL, clear, colorless
storage temp.
2-8°C
SMILES string
F[B-](F)(F)F.CC[O+](CC)CC
InChI
1S/C6H15O.BF4/c1-4-7(5-2)6-3;2-1(3,4)5/h4-6H2,1-3H3;/q+1;-1
InChI key
IYDQMLLDOVRSJJ-UHFFFAOYSA-N
Application
- To prepare amino esters by reacting with lactams followed by hydrolysis.
- In the preparation of substituted imidazolines from aziridines and nitriles via [3+2]-cycloaddition reaction.
- For the N-alkylation of a series of N-arylsulfonyl-α-amino acid methyl esters having variable substituents at 4th position of the sulfonamide aromatic ring.
Other Notes
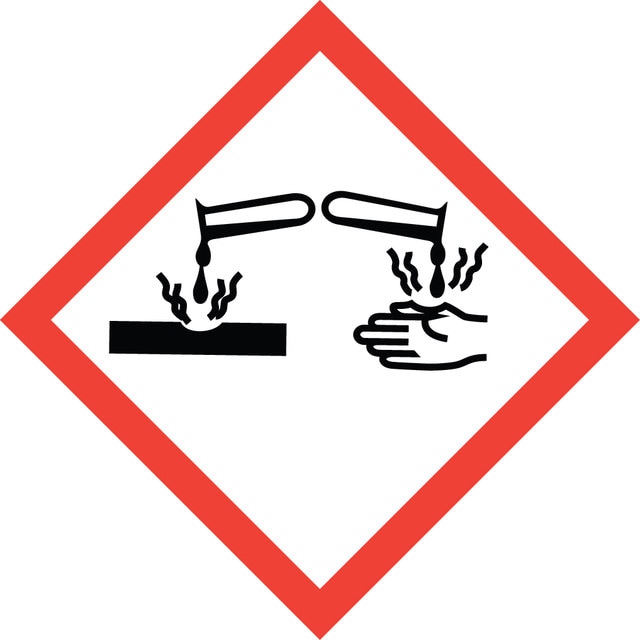
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
supp_hazards
Storage Class
8A - Combustible, corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service