932205
RuPhos ChemBeads
Synonym(s):
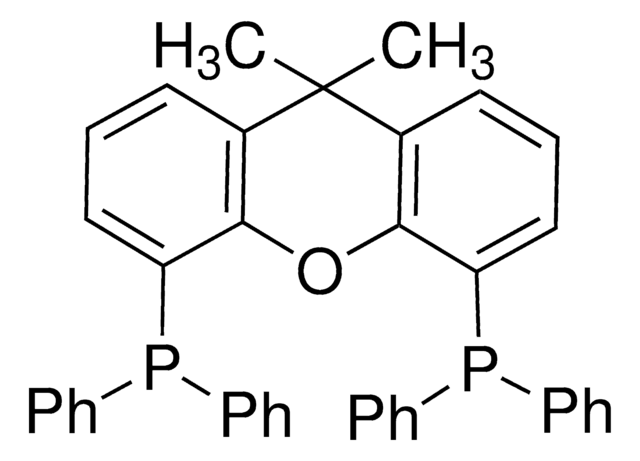
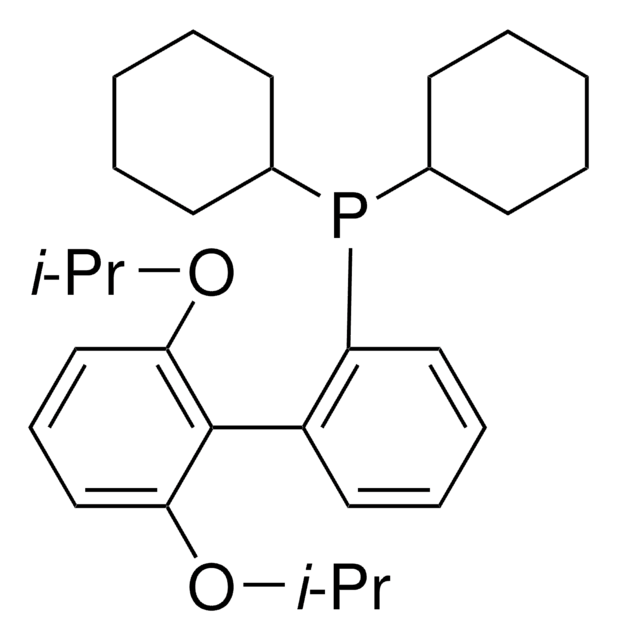
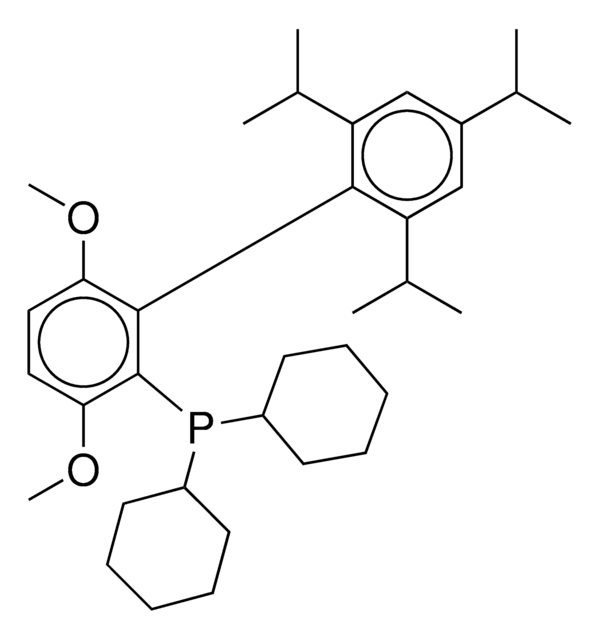
2-Dicyclohexylphosphino-2′,6′-diisopropoxybiphenyl
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C30H43O2P
CAS Number:
Molecular Weight:
466.64
UNSPSC Code:
12352112
Recommended Products
form
solid
Quality Level
storage temp.
2-8°C
InChI
1S/C30H43O2P/c1-22(2)31-27-19-13-20-28(32-23(3)4)30(27)26-18-11-12-21-29(26)33(24-14-7-5-8-15-24)25-16-9-6-10-17-25/h11-13,18-25H,5-10,14-17H2,1-4H3
InChI key
MXFYYFVVIIWKFE-UHFFFAOYSA-N
Related Categories
Application
Bulky phosphine ligand used in a palladium-catalyzed cross-coupling of aminoethyltrifluoroborates with electron-poor aryl bromides.
ChemBeads are chemical coated glass beads. ChemBeads offer improved flowability and chemical uniformity perfect for automated solid dispensing and high-throughput experimentation. The method of creating ChemBeads uses no other chemicals or surfactants allowing the user to accurately dispense sub-milligram amounts of chemical.
Learn more about ChemBeads products
Product is also available as neat precatalyst (663131)
ChemBeads are chemical coated glass beads. ChemBeads offer improved flowability and chemical uniformity perfect for automated solid dispensing and high-throughput experimentation. The method of creating ChemBeads uses no other chemicals or surfactants allowing the user to accurately dispense sub-milligram amounts of chemical.
Learn more about ChemBeads products
Product is also available as neat precatalyst (663131)
Other Notes
High-Throughput Reaction Screening with Nanomoles of Solid Reagents Coated on Glass Beads
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
related product
Product No.
Description
Pricing
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Versatile Methods to Dispense Submilligram Quantities of Solids Using Chemical-Coated Beads for High-Throughput Experimentation
Martin, et al.
Organic Process Research & Development, 23, 1900?1907-1900?1907 (2019)
Gary A Molander et al.
Organic letters, 9(2), 203-206 (2007-01-16)
A set of phenethylamines has been successfully prepared via Suzuki-Miyaura cross-coupling of diverse potassium beta-aminoethyltrifluoroborates with aryl halides. The potassium beta-aminoethyltrifluoroborates were easily prepared via hydroboration of enamine and enamide precursors. [reaction: see text].
Ana L Aguirre et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 27(51), 12981-12986 (2021-07-08)
High-throughput experimentation (HTE) methods are central to modern medicinal chemistry. While many HTE approaches to C-N and Csp2 -Csp2 bonds are available, options for Csp2 -Csp3 bonds are limited. We report here how the adaptation of nickel-catalyzed cross-electrophile coupling of
David S Surry et al.
Angewandte Chemie (International ed. in English), 47(34), 6338-6361 (2008-07-30)
Palladium-catalyzed amination reactions of aryl halides have undergone rapid development in the last 12 years, largely driven by the implementation of new classes of ligands. Biaryl phosphanes have proven to provide especially active catalysts in this context. This Review discusses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service