B1170000
Busulfan
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
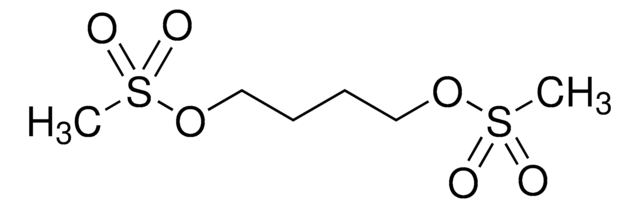
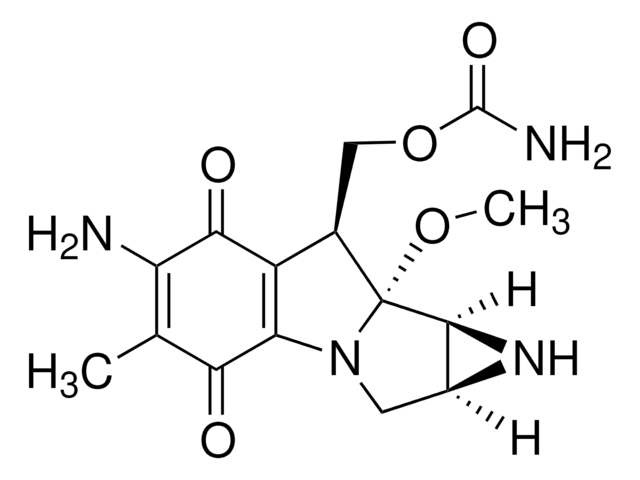
Busulfan, 1,4-Butanediol dimethanesulfonate
About This Item
Recommended Products
grade
pharmaceutical primary standard
API family
busulfan
manufacturer/tradename
EDQM
mp
114-117 °C (lit.)
application(s)
pharmaceutical (small molecule)
format
neat
SMILES string
CS(=O)(=O)OCCCCOS(C)(=O)=O
InChI
1S/C6H14O6S2/c1-13(7,8)11-5-3-4-6-12-14(2,9)10/h3-6H2,1-2H3
InChI key
COVZYZSDYWQREU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Packaging
Other Notes
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Carc. 1A - Muta. 1B - Repr. 1B
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service