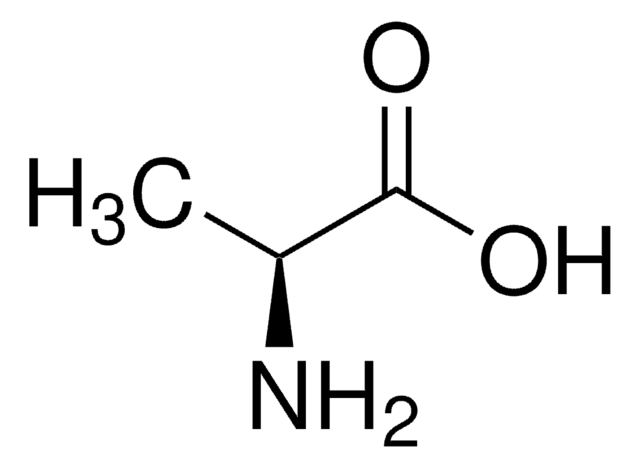
V0500
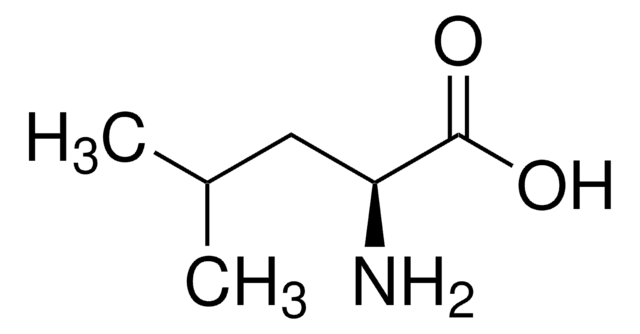
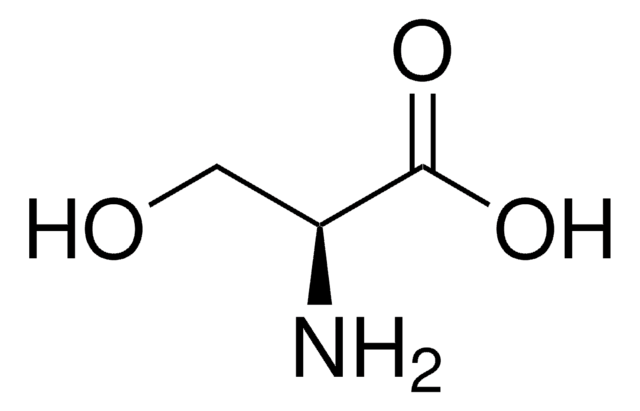
L-Valine
≥98% (HPLC), suitable for thin layer chromatography (TLC)
Synonym(s):
(S)-α-Aminoisovaleric acid, L-2-Amino-3-methylbutanoic acid
About This Item
Recommended Products
product name
L-Valine, reagent grade, ≥98% (HPLC)
grade
reagent grade
Quality Level
assay
≥98% (HPLC)
form
powder
technique(s)
thin layer chromatography (TLC): suitable
color
white
mp
295-300 °C (subl.) (lit.)
solubility
H2O: 50 mg/mL, clear, colorless
SMILES string
CC(C)[C@H](N)C(O)=O
InChI
1S/C5H11NO2/c1-3(2)4(6)5(7)8/h3-4H,6H2,1-2H3,(H,7,8)/t4-/m0/s1
InChI key
KZSNJWFQEVHDMF-BYPYZUCNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- L-Valine has been used as a standard in thin layer chromatography.
- It has been used as a supplement in Grace′s antheraea medium for the culture of cells and tissue from invertebrates.
- It has been used in the preparation of starvation media to study the role of relA gene in antibiotic resistance in E. coli.
Biochem/physiol Actions
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service