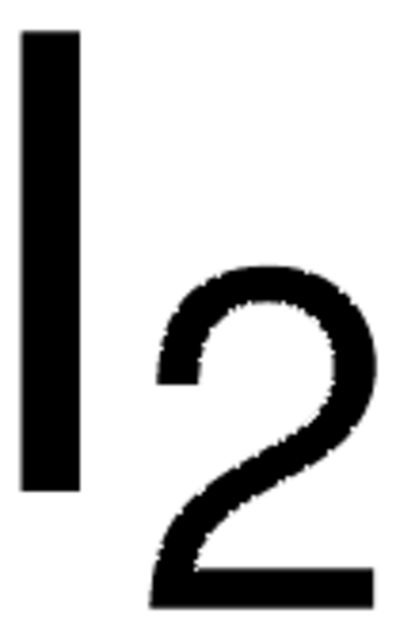208221
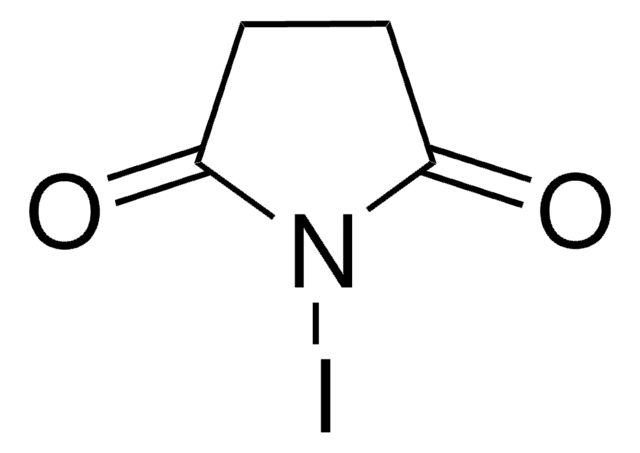
Iodine monochloride
reagent grade, ≥95%
Synonym(s):
Chloroiodide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
ICl
CAS Number:
Molecular Weight:
162.36
EC Number:
MDL number:
UNSPSC Code:
12352300
PubChem Substance ID:
NACRES:
NA.21
grade
reagent grade
Quality Level
assay
≥95%
form
solid or liquid
bp
97.4 °C (lit.)
density
3.24 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
ClI
InChI
1S/ClI/c1-2
InChI key
QZRGKCOWNLSUDK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Iodine monochloride is an interhalogen compound. It forms various complexes with ethyl, isopropyl and t-butylbenzenes. Electrically conducting solution of ICl is obtained on dissolution of ICl in polar solvents. ICl is a green oxidizing agent and participates in the following transformations:
- aldose hemiacetals to the corresponding aldose lactones
- diarylmethanols to the corresponding diarylmethanones
- arylalkylmethanols to the corresponding arylalkylmethanones
- dialkylmethanols to the corresponding dialkylmethanones
Application
Iodine monochloride (ICl) may be employed as a reagent in the following processes:
- Halogenation of methoxy and dimethoxybenzenes
- Synthesis of flavones.
- Preparation of 1-naphthaldehydes.
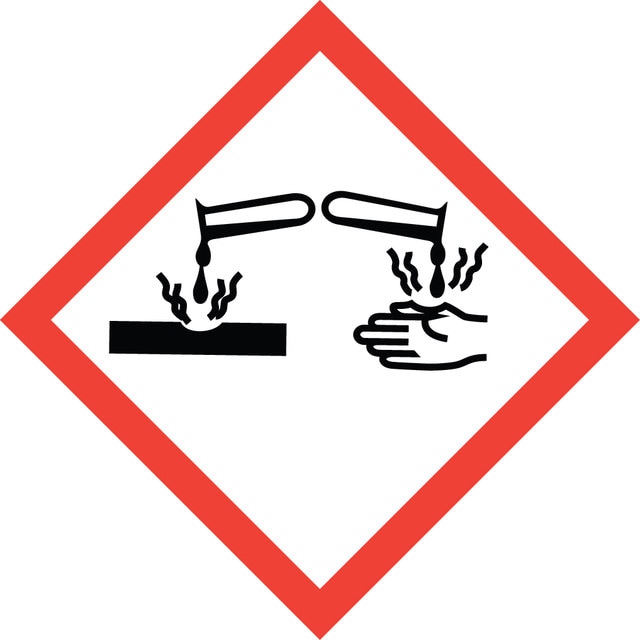
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Iodine Monochloride (ICl) as a Highly Efficient, Green Oxidant for the Oxidation of Alcohols to Corresponding Carbonyl Compounds
Wei, Peng, et al.
Synthetic Communications, 45.12, 1457-1470 (2015)
Synthesis of 1-naphthaldehydes via the cascade reactions of 1-phenylpent-4-yn-2-ols promoted by iodine monochloride
Li B, et al.
Tetrahedron Letters, 57.17, 1843-1846 (2016)
A mild and convenient procedure for conversion of alkenes into alkyl iodides via reaction of iodine monochloride with organoboranes.
Kabalka GW and Gooch III EE.
The Journal of Organic Chemistry, 45(18), 3578-3580 (1980)
Cation radicals as intermediates in aromatic halogenation with iodine monochloride: solvent and salt effects on the competition between chlorination and iodination.
Hubig SM, et al.
The Journal of Organic Chemistry, 59(21), 6233-6244 (1994)
Ultrasonic-assisted synthesis of flavones by oxidative cyclization of 2'-hydroxychalcones using iodine monochloride.
Lahyani A and Trabelsi M.
Ultrasonics Sonochemistry, 31, 626-630 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service