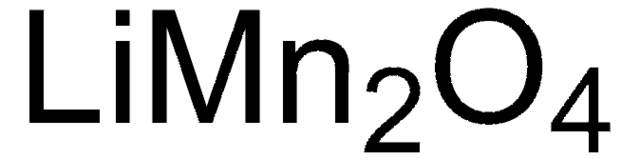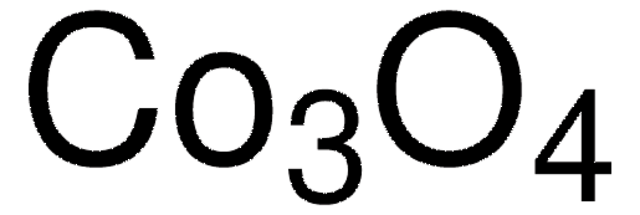310700
Manganese(IV) oxide
10 μm, reagent grade, ≥90%
Synonym(s):
Manganese dioxide
About This Item
grade
reagent grade
Quality Level
assay
≥90%
form
powder
particle size
10 μm
mp
535 °C (dec.) (lit.)
SMILES string
O=[Mn]=O
InChI
1S/Mn.2O
InChI key
NUJOXMJBOLGQSY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- High-oxidation-state 3d metal complexes: Explores the catalytic properties of manganese(IV) oxide within high-oxidation-state complexes for advanced organic synthesis, demonstrating its critical role in accelerating chemical reactions and enhancing yield efficiencies, beneficial for pharmaceutical and chemical industries (Cheng J et al., 2018).
- Synthesis and properties of manganese complexes: Details the synthesis of new manganese complexes that demonstrate unique redox properties, useful for understanding electron transfer processes in various chemical and environmental contexts (Baffert C et al., 2002).
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - STOT RE 2 Inhalation
target_organs
Brain
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 1
flash_point_f
does not flash
flash_point_c
does not flash
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service