18507
Atto 565 maleimide
BioReagent, suitable for fluorescence
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
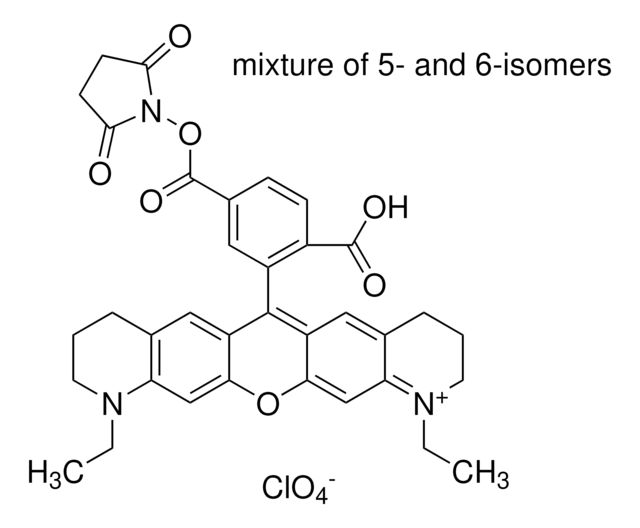
C37H37ClN4O10
Molecular Weight:
733.16
MDL number:
UNSPSC Code:
12352111
NACRES:
NA.32
Recommended Products
product line
BioReagent
Quality Level
assay
>90.0% (HPLC)
form
powder
manufacturer/tradename
ATTO-TEC GmbH
transmittance
254 nm
565 nm
fluorescence
λex 563 nm; λem 592 nm in 0.1 M phosphate pH 7.0
λ
(ethanol with 0.1% trifluoroacetic acid)
UV absorption
λ: 560-566 nm Amax
suitability
corresponds for coupling to thiols
suitable for fluorescence
storage temp.
−20°C
Application
Atto 565 is a new label with high molecular absorption (120.000) and quantum yield (0.9) as well as sufficient stoke′s shift (excitation maximum 563 nm, emission maximum 592 nm). Due to an insignificant triplet formation rate it is well suited for single molecule detection applications. Maleimides are well suited for coupling to thiol groups. This is similar to iodacetamides, but maleimides do react more thiol selective. They do not show significant reaction with histidine or methionine. Hydrolysis of maleimides to a mixture of isomeric nonreactive maleamic acids can compete significantly with thiol modification, particularly above pH 8. Maleimides may be used for labeling of amines, which usually requires a higher pH than reaction of maleimides with thiols.
Legal Information
This product is for Research use only. In case of intended commercialization, please contact the IP-holder (ATTO-TEC GmbH, Germany) for licensing.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shadi Ferdosi et al.
Journal of proteome research, 17(1), 543-558 (2017-11-14)
Glycans represent a promising but only marginally accessed source of cancer markers. We previously reported the development of a molecularly bottom-up approach to plasma and serum (P/S) glycomics based on glycan linkage analysis that captures features such as α2-6 sialylation
Lukasz Krzemiński et al.
The journal of physical chemistry. B, 115(43), 12607-12614 (2011-09-24)
Recently, studies have been reported in which fluorescently labeled redox proteins have been studied with a combination of spectroscopy and electrochemistry. In order to understand the effect of the dye on the protein-electrode interaction, voltammetry and surface analysis have been
Suman Lata et al.
Journal of the American Chemical Society, 128(7), 2365-2372 (2006-02-16)
Labeling of proteins with fluorescent dyes offers powerful means for monitoring protein interactions in vitro and in live cells. Only a few techniques for noncovalent fluorescence labeling with well-defined localization of the attached dye are currently available. Here, we present
Thiol reactive probes and chemosensors.
Peng H, Chen W, Cheng Y, et al.
Sensors, 12, 15907-15946 (2012)
Łukasz Krzemiński et al.
Journal of the American Chemical Society, 133(38), 15085-15093 (2011-08-26)
A combined fluorescence and electrochemical method is described that is used to simultaneously monitor the type-1 copper oxidation state and the nitrite turnover rate of a nitrite reductase (NiR) from Alcaligenes faecalis S-6. The catalytic activity of NiR is measured
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service