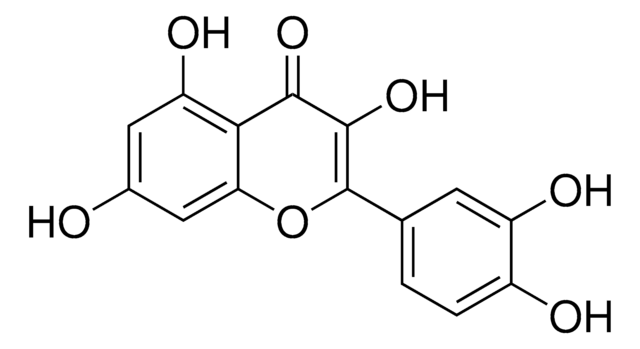
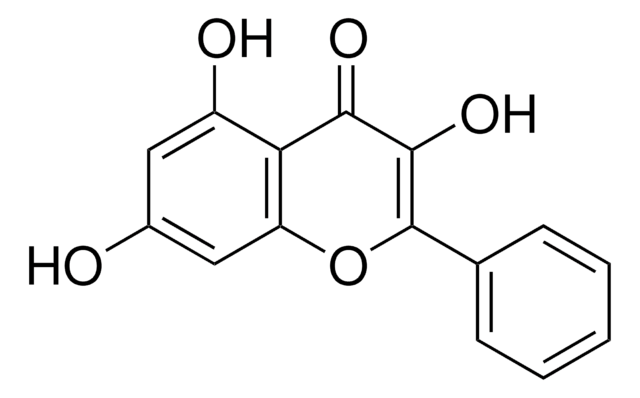
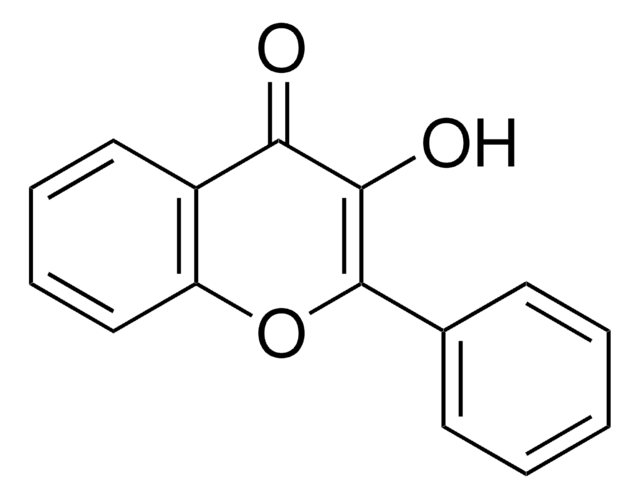
282200
Galangin
autophagy inducing flavonoid
Synonym(s):
3,5,7-Trihydroxyflavone, Norizalpinin
About This Item
Recommended Products
Quality Level
assay
≥95% (HPLC)
form
powder
color
yellow
mp
214-215 °C (lit.)
SMILES string
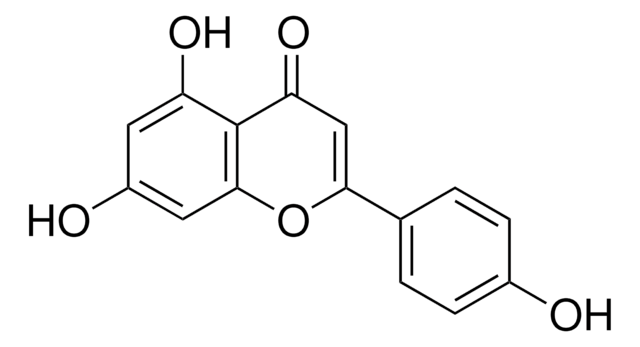
Oc1cc(O)c2C(=O)C(O)=C(Oc2c1)c3ccccc3
InChI
1S/C15H10O5/c16-9-6-10(17)12-11(7-9)20-15(14(19)13(12)18)8-4-2-1-3-5-8/h1-7,16-17,19H
InChI key
VCCRNZQBSJXYJD-UHFFFAOYSA-N
Gene Information
human ... ADORA2A(135) , ADORA3(140) , CYP1A2(1544)
rat ... Adora1(29290) , Adora2a(25369)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as a test drug to test its ameliorative effect in a rodent model of cisplatin-induced nephrotoxicity
- to test its effect on the differentiation of 3T3-L1 preadipocyte cells into adipocytes
- as an internal standard in nuclear magnetic resonance spectroscopy and mass spectroscopy
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Protocols
ASTM D6526: GC Analysis of Impurities in Toluene on SLB®-IL100, 60 m Column
US EPA Method 8260 describes the analysis of volatile organic compounds in solid wastes and ground waters. This application illustrates the analysis of many compounds commonly analyzed by this method using purge and trap coupled to GC-MS.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service