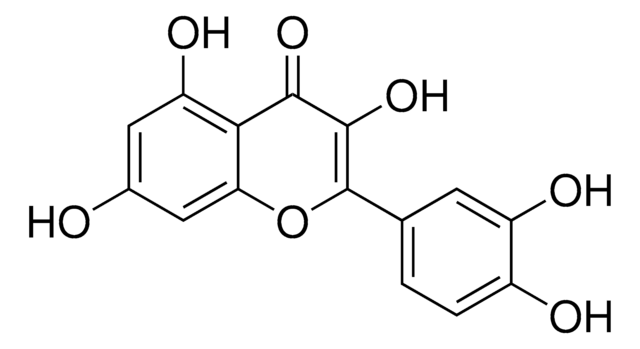
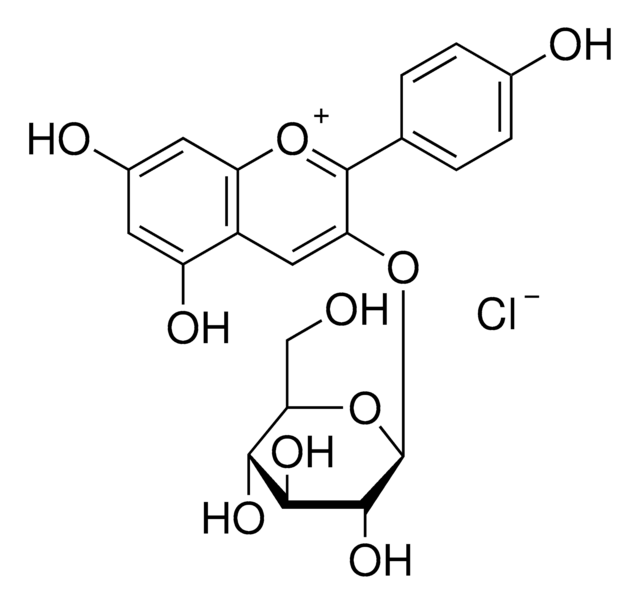
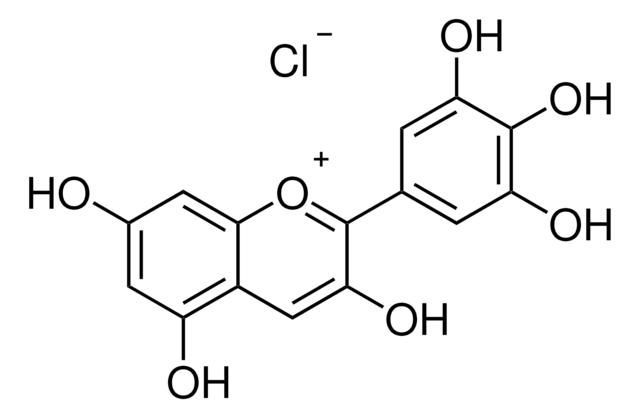
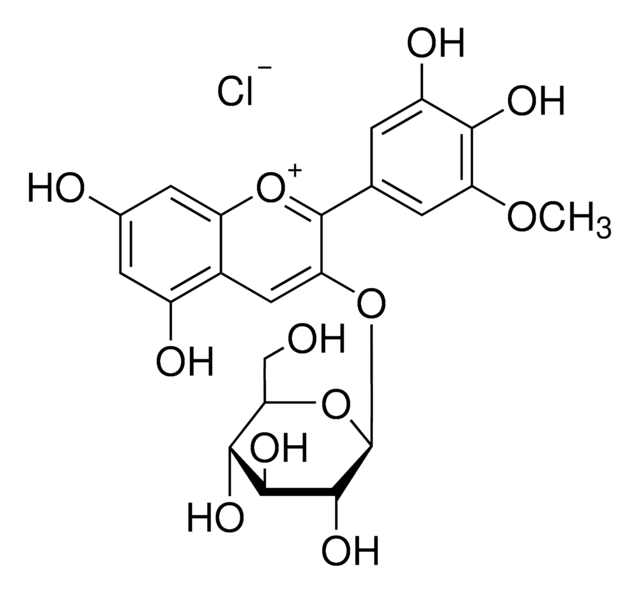
40796
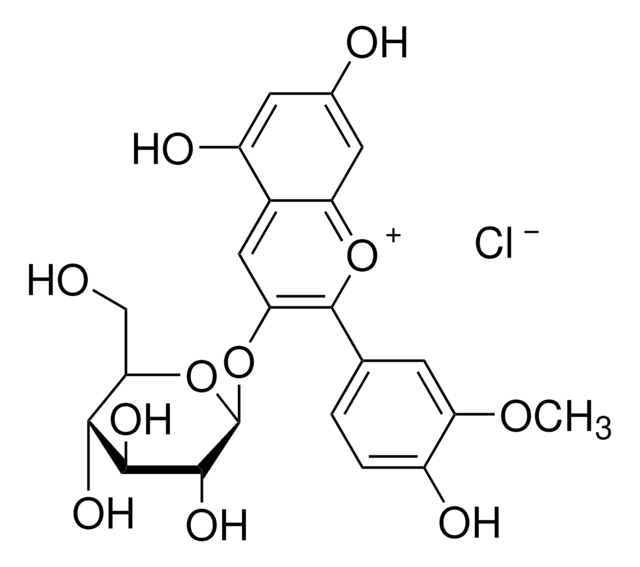
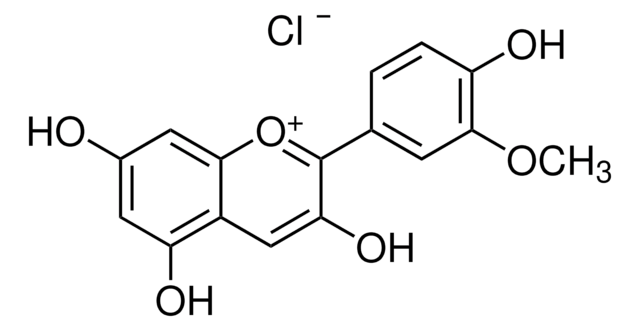
Peonidin 3-O-glucoside chloride
≥95% (HPLC)
Synonym(s):
3-(Glucosyloxy)-4′,5,7-trihydroxy-3′-methoxyflavylium chloride, Glucopeonidin chloride
About This Item
Recommended Products
biological source
synthetic
Quality Level
assay
≥95% (HPLC)
form
powder
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
−20°C
SMILES string
[Cl-].COc1cc(ccc1O)-c2[o+]c3cc(O)cc(O)c3cc2O[C@@H]4O[C@H](CO)[C@@H](O)[C@H](O)[C@H]4O
InChI
1S/C22H22O11.ClH/c1-30-15-4-9(2-3-12(15)25)21-16(7-11-13(26)5-10(24)6-14(11)31-21)32-22-20(29)19(28)18(27)17(8-23)33-22;/h2-7,17-20,22-23,27-29H,8H2,1H3,(H2-,24,25,26);1H/t17-,18-,19+,20-,22-;/m1./s1
InChI key
VDTNZDSOEFSAIZ-VXZFYHBOSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
Packaging
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
-glucoside chloride; Malvidin 3-glucoside; Delphinidin 3-(6-acetylglucoside); Cyanidin 3-(6-acetylglucoside); Petunidin 3-(6-acetylglucoside); Peonidin 3-(6-acetylglucoside); Malvidin 3-(6-acetylglucoside); Malvidin 3-(6-caffeoylglucoside); Petunidin 3-(6-cumarylglucoside); Peonidin 3-(6-cumarylglucoside); Malvidin 3-(6-cumarylglucoside)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service