50950
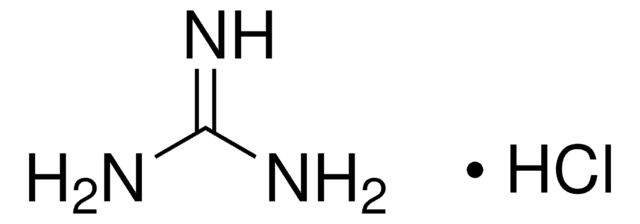
Guanidine hydrochloride
≥98%
Synonym(s):
Aminoformamidine hydrochloride, Aminomethanamidine hydrochloride, Guanidinium chloride
About This Item
Recommended Products
Quality Level
assay
≥98%
form
powder or crystals
storage condition
(Tightly closed. Dry. )
color
colorless to white
pH
(25 °C, 4.6 - 6/573 g/L)
mp
180-185 °C (lit.)
solubility
H2O: 6 M, clear (100 Hazen)
density
1.3 g/cm3 (lit.)
SMILES string
Cl[H].NC(N)=N
InChI
1S/CH5N3.ClH/c2-1(3)4;/h(H5,2,3,4);1H
InChI key
PJJJBBJSCAKJQF-UHFFFAOYSA-N
Gene Information
human ... KCNA1(3736) , KCNA10(3744) , KCNA2(3737) , KCNA3(3738) , KCNA4(3739) , KCNA5(3741) , KCNA6(3742) , KCNA7(3743) , KCNB1(3745) , KCNB2(9312) , KCNC1(3746) , KCNC2(3747) , KCNC3(3748) , KCNC4(3749) , KCND1(3750) , KCND2(3751) , KCND3(3752) , KCNF1(3754) , KCNG1(3755) , KCNG2(26251) , KCNG3(170850) , KCNG4(93107) , KCNH1(3756) , KCNH2(3757) , KCNH3(23416) , KCNH4(23415) , KCNH5(27133) , KCNH6(81033) , KCNH7(90134) , KCNH8(131096) , KCNQ1(3784) , KCNQ2(3785) , KCNQ3(3786) , KCNQ4(9132) , KCNQ5(56479) , KCNS1(3787) , KCNS2(3788) , KCNS3(3790) , KCNV1(27012) , KCNV2(169522)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
At lower concentrations, guanidine hydrochloride has the intriguing ability to promote the refolding of denatured proteins, aiding in protein renaturation studies. In RNA extraction, it acts as a strong denaturant, disrupting cell structures and ensuring the integrity of extracted RNA by inactivating RNA enzymes. Overall, guanidine hydrochloride′s denaturing and renaturing properties make it an essential reagent for various cell biology applications, including protein purification, nucleic acid isolation, and protein refolding studies.
Application
- for lysing homogenized brain tissue
- in the preparation of incubation buffer for Ni-sepharose protein binding, purification, propionylation, and on-bead digestion to minimize nonspecific binding to the affinity resin
- in the preparation of solutions/extraction buffer to extract soluble protein from the human tissue
Biochem/physiol Actions
Features and Benefits
Other Notes
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service