60010
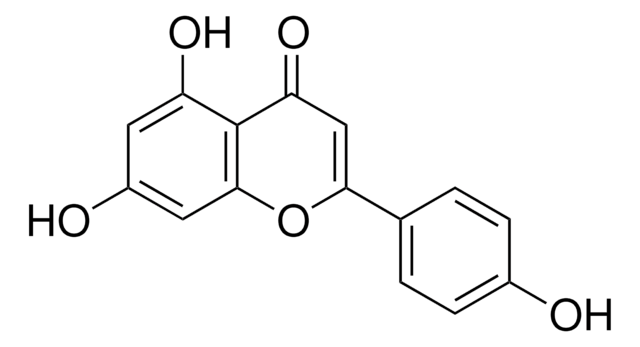
Kaempferol
≥97.0% (HPLC)
Synonym(s):
3,4′,5,7-Tetrahydroxyflavone, 3,5,7-Trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, Robigenin
About This Item
Recommended Products
Quality Level
assay
≥97.0% (HPLC)
impurities
≤12% water
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
SMILES string
Oc1ccc(cc1)C2=C(O)C(=O)c3c(O)cc(O)cc3O2
InChI
1S/C15H10O6/c16-8-3-1-7(2-4-8)15-14(20)13(19)12-10(18)5-9(17)6-11(12)21-15/h1-6,16-18,20H
InChI key
IYRMWMYZSQPJKC-UHFFFAOYSA-N
Gene Information
human ... CDC2(983) , CDK5(1020) , CDK6(1021) , CYP1A2(1544) , CYP2C9(1559) , GSK3A(2931)
mouse ... Hexa(15211)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- to test its effect on osteoblast proliferation, migration, and differentiation in mouse pre-osteoblast cell line (MC3T3-E1 cells)
- as an antioxidant and a hepatoprotective agent against zearalenone (ZEA)-induced oxidative stress and apoptosis in HEPG2 cells
- as a neuroprotective agent against cadmium chloride (CdCl2) induced hippocampal damage and memory deficits in rats
- as an anti-inflammatory agent, together with apigenin, for stem-cell therapy on knee osteoarthritic male rats
Biochem/physiol Actions
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Antioxidants protect biological systems from oxidative damage produced by oxygen-containing free radicals and from redoxactive transition metal ions such as iron, copper, and cadmium.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service