F7250
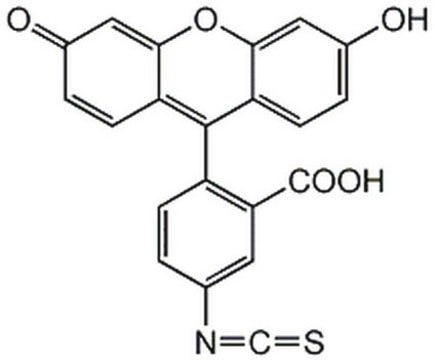
Fluorescein isothiocyanate isomer I
suitable for protein labeling, ≥90% (HPLC), powder
Synonym(s):
FITC, Fluorescein 5-isothiocyanate
About This Item
Recommended Products
Quality Level
assay
≥90% (HPLC)
form
powder
color
orange to dark orange
mp
>360 °C (lit.)
solubility
acetone: 1 mg/mL
fluorescence
λex 492 nm; λem 518 nm (green)
suitability
suitable for protein labeling
application(s)
diagnostic assay manufacturing
hematology
histology
storage temp.
2-8°C
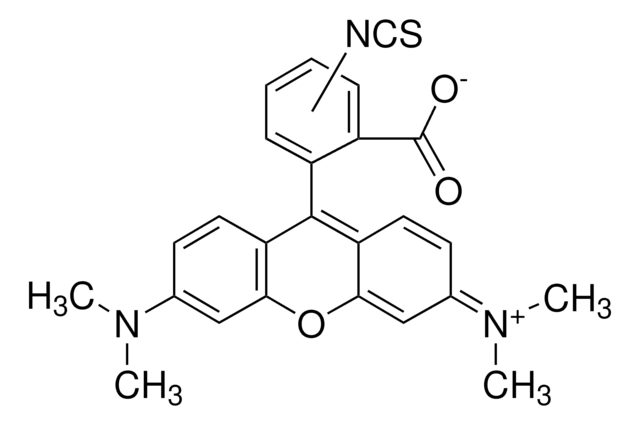
SMILES string
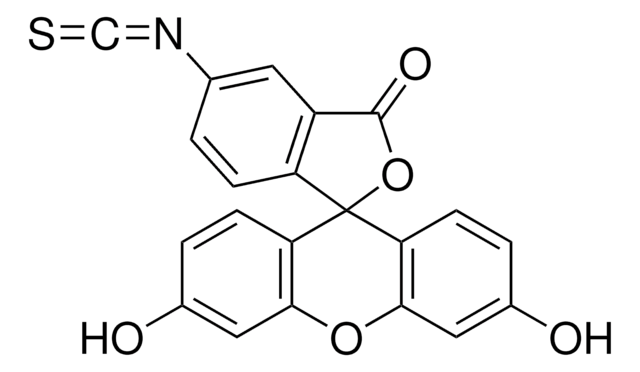
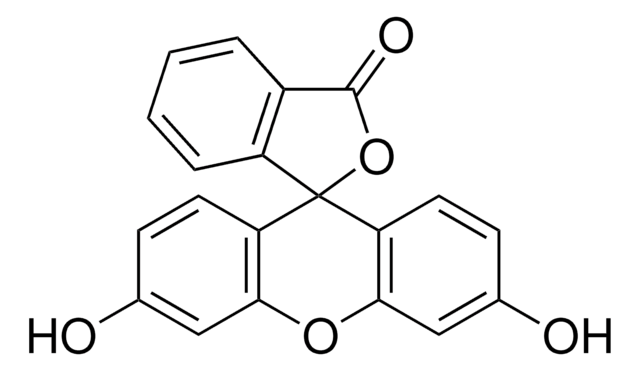
Oc1ccc2c(Oc3cc(O)ccc3C24OC(=O)c5cc(ccc45)N=C=S)c1
InChI
1S/C21H11NO5S/c23-12-2-5-16-18(8-12)26-19-9-13(24)3-6-17(19)21(16)15-4-1-11(22-10-28)7-14(15)20(25)27-21/h1-9,23-24H
InChI key
MHMNJMPURVTYEJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
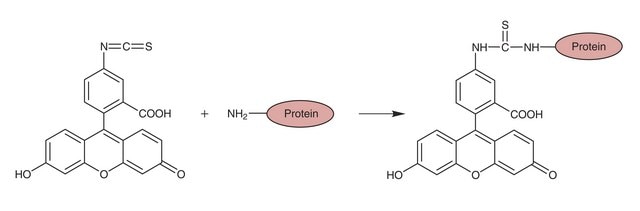
It is widely used to attach a fluorescent label to proteins via the amine group. The isothiocyanate group reacts with amino terminal and primary amines in proteins. It has been used for the labeling of proteins including antibodies and lectins.
Fluorescein isothiocyanate isomer I has been proposed as a contact sensitizer.
Application
Biological applications include use as a fluorescent labeling reagent for proteins, a fluorescent reagent for protein tracing, and a reagent in the fluorescent antibody technique for the rapid identification of pathogens. It may be employed as the derivatization reagent for amphetamine, methamphetamine, 3,4-methylenedioxymethamphetamine and P-phenylethylamine in human urine during their capillary electrophoretic (CE) determination. It may be used for the preparation of fluorescent antibodies. It was employed for in vitro sensitization studies.
signalword
Danger
hcodes
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Nitric oxide (NO) as a signal transporter in neurons, endothelial cells and in the immune system.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service