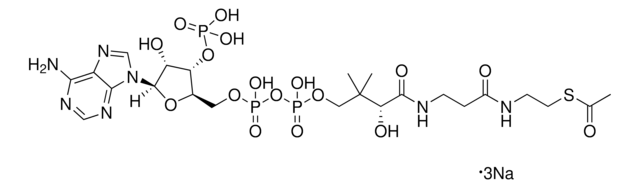
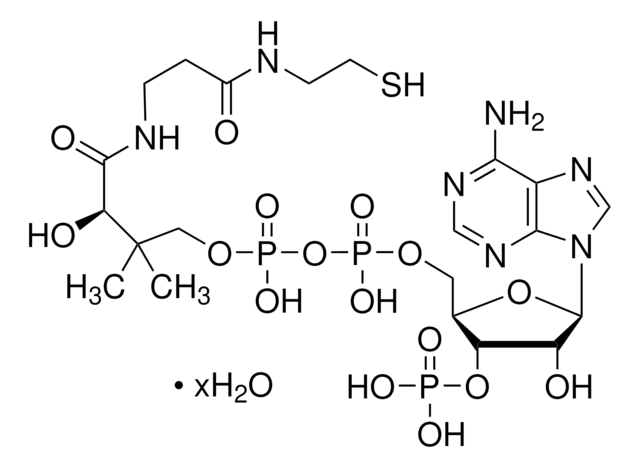
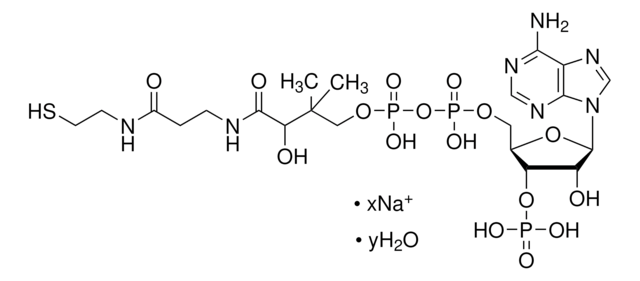
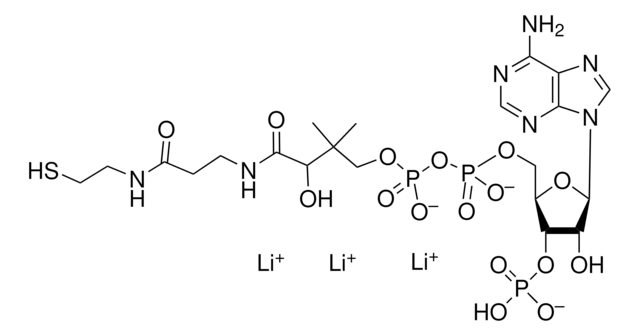
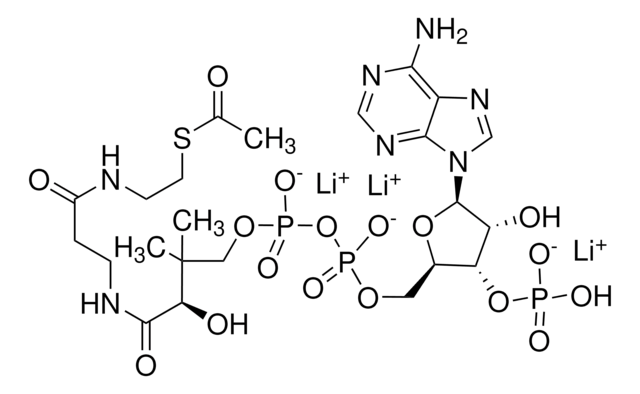
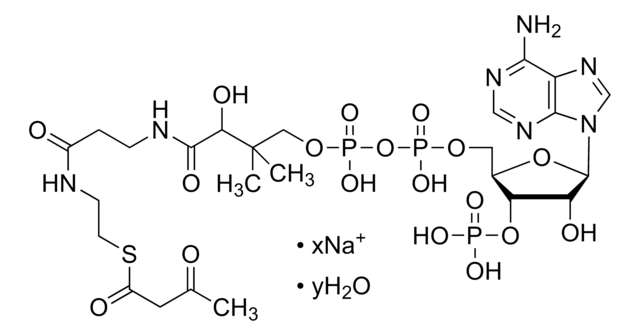
A1765
S-Acetyl-coenzyme A synthetase from baker′s yeast (S. cerevisiae)
lyophilized powder, ≥3 units/mg protein
Synonym(s):
Acetate CoA ligase (AMP forming), Acetate thiokinase
About This Item
Recommended Products
form
lyophilized powder
Quality Level
specific activity
≥3 units/mg protein
composition
Protein, 10-30% biuret
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Biochem/physiol Actions
Packaging
Unit Definition
Physical form
signalword
Danger
hcodes
pcodes
Hazard Classifications
Resp. Sens. 1
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Enzyme Reagent Coenzyme A (CoA, CoASH or HSCoA) is the key cofactor in first step of the TCA cycle, responsible for transferring the acetyl group from pyruvate oxidation to oxaloacetate yielding citrate. Available through Sigma-Aldrich online.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service