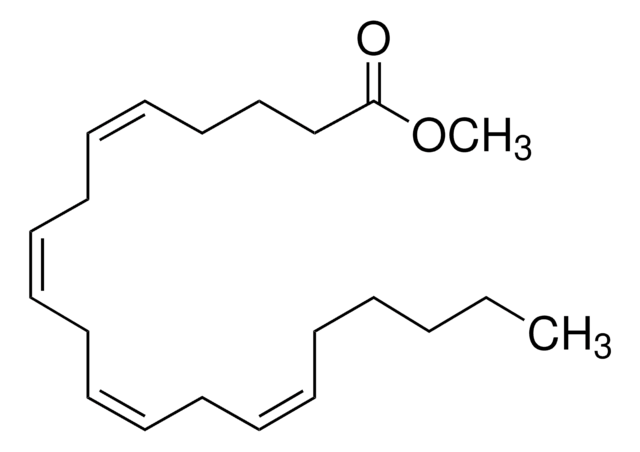
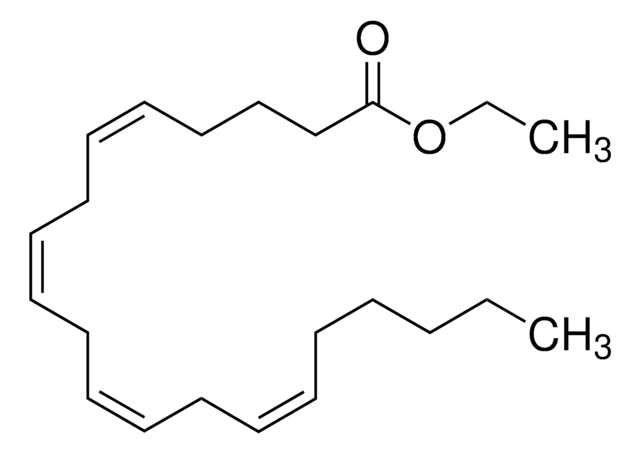
A9298
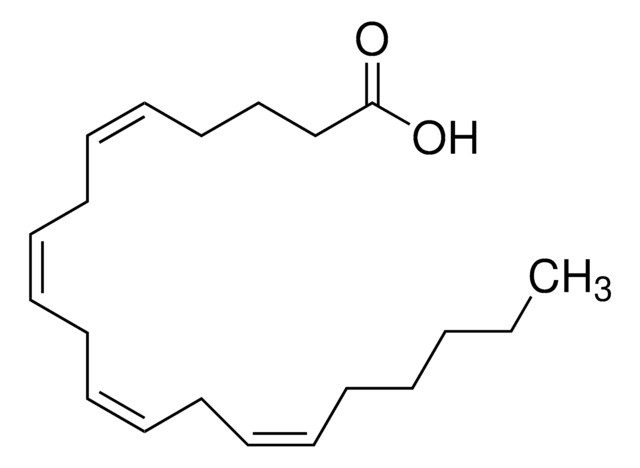
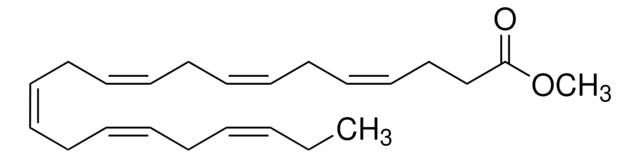
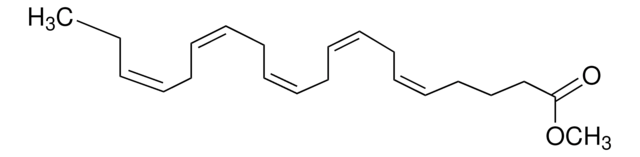
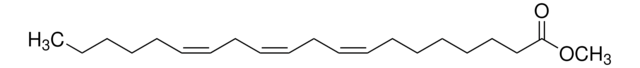
Methyl arachidonate
≥99% (GC)
Synonym(s):
Arachidonic acid methyl ester
About This Item
Recommended Products
biological source
Mortierella alpina
Quality Level
assay
≥99% (GC)
form
liquid
functional group
ester
lipid type
omega FAs
shipped in
dry ice
storage temp.
−20°C
SMILES string
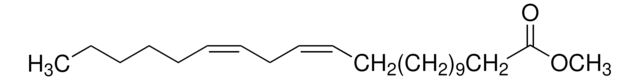
CCCCC\C=C/C\C=C/C\C=C/C\C=C/CCCC(=O)OC
InChI
1S/C21H34O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21(22)23-2/h7-8,10-11,13-14,16-17H,3-6,9,12,15,18-20H2,1-2H3/b8-7-,11-10-,14-13-,17-16-
InChI key
OFIDNKMQBYGNIW-ZKWNWVNESA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Lipid peroxidation products mediate the formation of 8-hydroxydeoxyguanosine in DNA.: This article explores the role of lipid peroxidation products, specifically those involving methyl arachidonate, in causing oxidative DNA damage, indicating the compound′s influence on cellular aging and disease processes (Park JW et al., 1992).
Biochem/physiol Actions
Packaging
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
gc-analysis-of-a-37-component-fame-mix-g004278
GC Analysis of a 37-Component FAME Mix on Omegawax® (15 m x 0.10 mm I.D., 0.10 μm), Fast GC Analysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service