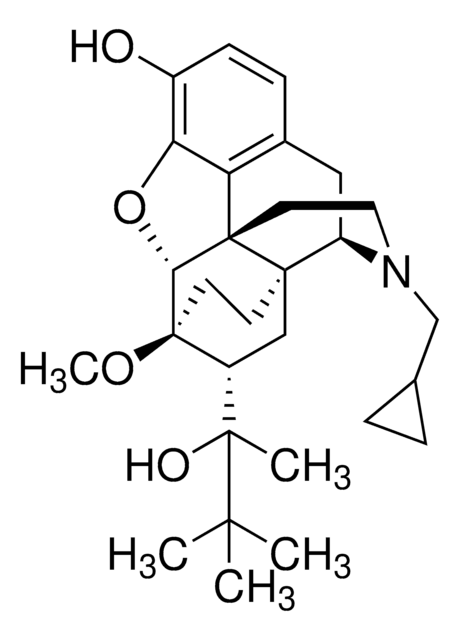
B8181
BID human
≥95% (SDS-PAGE), recombinant, expressed in E. coli, buffered aqueous solution
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
Biochem/physiol Actions
Regulates outer mitochondrial membrane permeability, pro-apoptotic, causes release of cytochrome c from mitochondrial intermembrane space to cytosol.
Other Notes
Human BID (195 amino acid residues) is a member of the Bcl-2 family (accession number NM 001196).
Physical form
Solution, 0.2 μm filtered, in 25 mM HEPES, pH 7.5, and 0.1 M KCl.
Analysis Note
Measured by its ability to induce cytochrome c release from isolated mouse liver mitochondria.
Storage Class
10 - Combustible liquids
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M C Wei et al.
Genes & development, 14(16), 2060-2071 (2000-08-19)
TNFR1/Fas engagement results in the cleavage of cytosolic BID to truncated tBID, which translocates to mitochondria. Immunodepletion and gene disruption indicate BID is required for cytochrome c release. Surprisingly, the three-dimensional structure of this BH3 domain-only molecule revealed two hydrophobic
J Zha et al.
Science (New York, N.Y.), 290(5497), 1761-1765 (2000-12-02)
Many apoptotic molecules relocate subcellularly in cells undergoing apoptosis. The pro-apoptotic protein BID underwent posttranslational (rather than classic cotranslational) N-myristoylation when cleavage by caspase 8 caused exposure of a glycine residue. N-myristoylation enabled the targeting of a complex of p7
X Luo et al.
Cell, 94(4), 481-490 (1998-09-04)
We report here the purification of a cytosolic protein that induces cytochrome c release from mitochondria in response to caspase-8, the apical caspase activated by cell surface death receptors such as Fas and TNF. Peptide mass fingerprinting identified this protein
H Li et al.
Cell, 94(4), 491-501 (1998-09-04)
We report here that BID, a BH3 domain-containing proapoptotic Bcl2 family member, is a specific proximal substrate of Casp8 in the Fas apoptotic signaling pathway. While full-length BID is localized in cytosol, truncated BID (tBID) translocates to mitochondria and thus
BCL-2 family members and the mitochondria in apoptosis.
A Gross et al.
Genes & development, 13(15), 1899-1911 (1999-08-13)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service