C0494
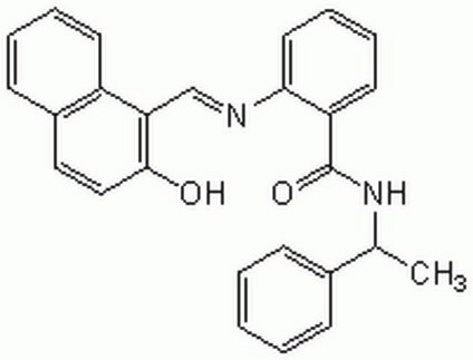
Cambinol
≥97% (HPLC), white, powder
Synonym(s):
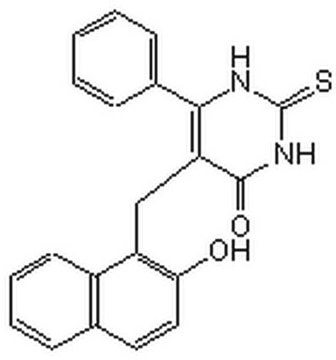
5-(2-Hydroxynaphthalen-1-ylmethyl)-6-phenyl-2-thioxo-2,3-dihydro-1H-pyrimidin-4-one, 5-[(2-hydroxy-1-naphthyl)methyl]-2-mercapto-6-phenyl-4(3H)-Pyrimidinone, NSC-1125476, Tetrahydro-5-[(2-hydroxy-1-naphthalenyl)methyl]-6-phenyl-2-thioxo-4(1H)-Pyrimidinone
About This Item
Recommended Products
Quality Level
assay
≥97% (HPLC)
form
powder
color
white
solubility
DMSO: ≥10 mg/mL
storage temp.
room temp
SMILES string
Oc1ccc2ccccc2c1CC3=C(NC(=S)NC3=O)c4ccccc4
InChI
1S/C21H16N2O2S/c24-18-11-10-13-6-4-5-9-15(13)16(18)12-17-19(14-7-2-1-3-8-14)22-21(26)23-20(17)25/h1-11,24H,12H2,(H2,22,23,25,26)
InChI key
RVNSQVIUFZVNAU-UHFFFAOYSA-N
Application
- as an inhibitor of sirutins (SIRT1 and 2) in hepatocarcinoma cell lines to test its effect on cellular viability and colony formation
- as a SIRT1 and SIRT2 inhibitor to test its effect on frizzled 7 (FZD7) expression in the breast cancer cells MDA-MB-231 and T-47D cells
- as a sirutin inhibitor in L929 cells to test its protective effect against tumor necrosis factor (TNF)-induced cell death
Biochem/physiol Actions
Features and Benefits
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Related Content
We offer a variety of small molecule research tools, such as transcription factor modulators, inhibitors of chromatin modifying enzymes, and agonists/antagonists for target identification and validation in gene regulation research; a selection of these research tools is shown below.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service