C0663
Acetylcholinesterase from human erythrocytes
buffered aqueous solution, ≥500 units/mg protein (BCA)
Synonym(s):
AChE, Acetylcholine acetylhydrolase
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Recommended Products
General description
Acetylcholinesterase (AChE) belongs to the carboxyl esterase family of enzymes. The erythrocyte AChE is membrane bound. AChE is mapped to human chromosome 7q22.1. It is enriched in aged erythrocytes.
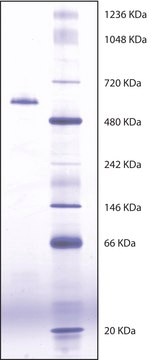
Predominantly exists as a tetrameric glycoprotein composed of disulfide-linked homodimers with a monomer MW of ~80 kDa.
Application
Acetylcholinesterase (AChE) from Sigma has been used in the structure-activity study of phosphoramido acid esters as inhibitors of AChE.
Acetylcholinesterase from human erythrocytes has been used in:
- cholinesterase inhibition assay for screening 4-aminoquinoline based compounds
- AChE activity assays to test the effect of positive allosteric modulators (PAMs)
- organophosphorus compounds based inhibition assay
Biochem/physiol Actions
Acetylcholinesterase (AChE) is regarded as a biomarker in neurotoxicity. It is a modulator of nitric oxide signal transduction pathway and marker of membrane integrity and aging. The levels of erythrocyte (RBC) AChE are affected on pesticide exposure and in hemolytic anemia. RBC AChE is a marker in Hirschsprung′s disease and inflammation.
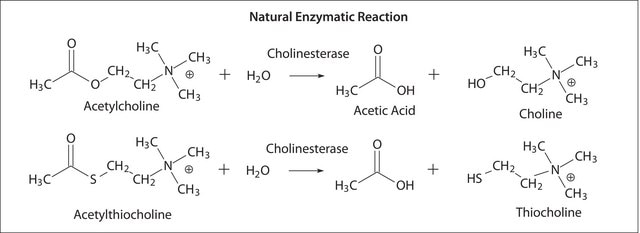
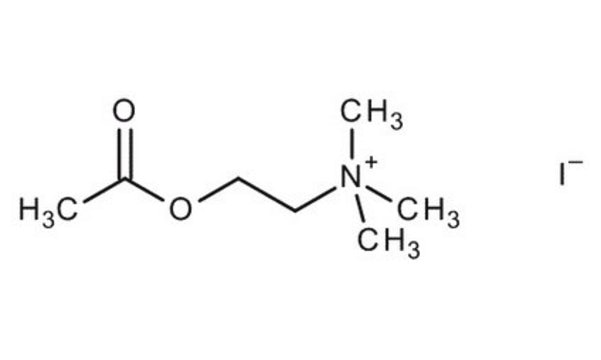
Acetylcholinesterase is the major in vivo degradative enzyme for acetylcholine. It converts acetylcholine and water to choline and acetic acid. Cholinesterases are inhibited by the natural carbamate alkaloid, eserine or physostigmine.
In blood there are two cholinesterases present: The erythrocyte associated enzyme, which is a true cholinesterase or acetylcholinesterase [(AChE) - E.C. 3.1.1.7], the serum associated enzyme, which is Pseudocholinesterase or Butyrylcholinesterase [(BuChE) - EC 3.1.1.8].
AChE is an ectoenzyme, anchored to the erythrocyte membrane via a GPI moiety.
AChE is an ectoenzyme, anchored to the erythrocyte membrane via a GPI moiety.
Unit Definition
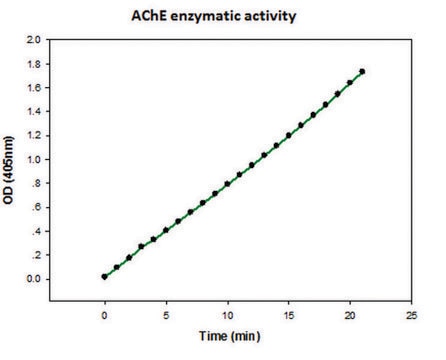
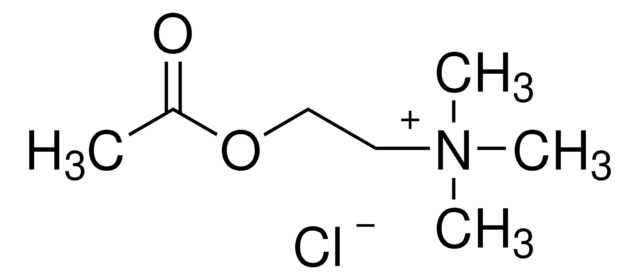
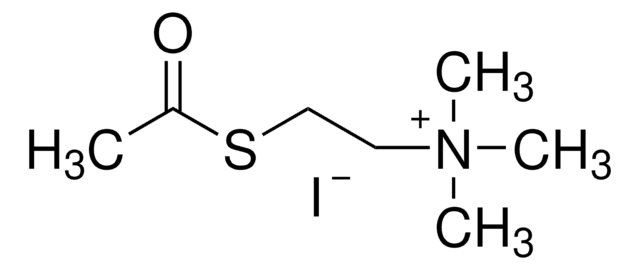
One unit will hydrolyze 1.0 μmole of acetylthiocholine iodide per min at pH 7.4 at 37 °C.
Physical form
Solution in 20 mM HEPES, pH 8.0, containing 0.1% TRITON® X-100
Preparation Note
The enzyme is the amphiphilic form extracted together with its GPI anchor with the aid of TRITON X-100 and purified by affinity chromatography.
Analysis Note
The activity obtained using acetylcholine as substrate is 30-100 times that obtained with butyrylcholine, using acetylcholinesterase from electric eel.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Saied Ghadimi et al.
Journal of enzyme inhibition and medicinal chemistry, 23(4), 556-561 (2008-07-31)
Phosphoramido acid esters (CH(3))(2)NP(O)X(p-OC(6)H(4)-CH(3)) (containing P-Cl (1), P-O (2), P-F (3), P-CN (5), and P-N (4,6) bonds, X for 2, 4 and 6 is OCH(3), (C(2)H(5))(2)N and morpholin) have been synthesized to investigate the structure-activity study of AChE enzyme inhibition
Identification of 4-aminoquinoline core for the design of new cholinesterase inhibitors
Chen Y, et al.
PeerJ, 4, e2140-e2140 (2016)
Variability of AChE, BChE, and ChAT genes in the late-onset form of Alzheimer's disease and relationships with response to treatment with Donepezil and Rivastigmine
Scacchi R, et al.
American Journal of Medical Genetics. Part B, Neuropsychiatric Genetics : the Official Publication of the International Society of Psychiatric Genetics, 150(4), 502-507 (2009)
High-Throughput Screening for Positive Allosteric Modulators Identified Potential Therapeutics against Acetylcholinesterase Inhibition
Chapleau RR, et al.
Journal of Biomolecular Screening, 20(9), 1142-1149 (2015)
Acetylcholinesterase from human erythrocytes as a surrogate biomarker of lead induced neurotoxicity
Gupta VK, et al.
Enzyme Research, 2015 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service