C2389
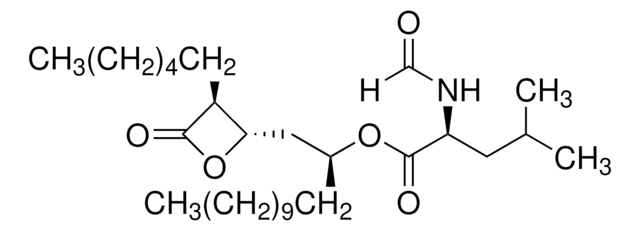
Cerulenin
≥98% (HPLC), from Cephalosporium caerulens
Synonym(s):
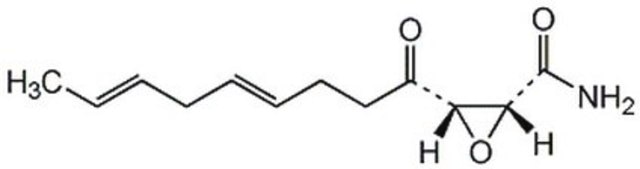
Helicocerin, (2R,3S,E,E)-2,3-Epoxy-4-oxo-7,10-dodecadienamide
About This Item
Recommended Products
biological source
Cephalosporium caerulens
Quality Level
assay
≥98% (HPLC)
form
powder
mp
93 °C
solubility
acetone: 19.60-20.40 mg/mL, clear to slightly hazy, colorless to yellow
antibiotic activity spectrum
fungi
mode of action
enzyme | inhibits
storage temp.
−20°C
SMILES string
C/C=C/C/C=C/CCC([C@@H]1[C@H](C(N)=O)O1)=O
InChI
1S/C13H18O3/c1-3-4-5-6-7-8-9-11(15)13-12(16-13)10(2)14/h3-4,6-7,12-13H,5,8-9H2,1-2H3/b4-3+,7-6+/t12-,13+/m0/s1
InChI key
PTNNGEBMCNMENY-JIVMHGEESA-N
Gene Information
human ... FASN(2194)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- as a fatty acid synthase inhibitor to study its effects on aldosterone-induced trained immunity
- as a blocker of fatty acid synthase to study its effects on the replication of severe acute respiratory syndrome coronavirus 2 (SARS-CoV2)
- as a supplement in yeast extract–peptone–dextrose/glycerol (YPD/G) agar plates for the isolation of cerulenin-resistant yeast strains
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The amount of cholesterol that is synthesized in the liver is tightly regulated by dietary cholesterol levels. LDL receptors regulate the cellular transport of lipid rich low density lipoprotein (LDL) particles.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Related Content
Discover Bioactive Small Molecules for Lipid Signaling Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service