E4143
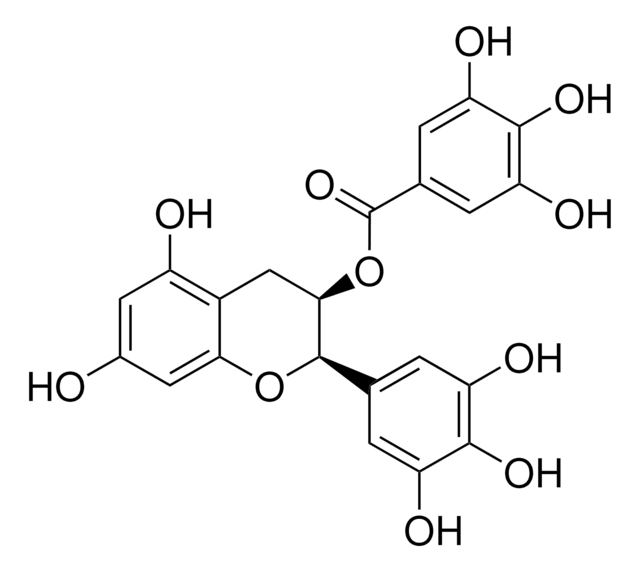
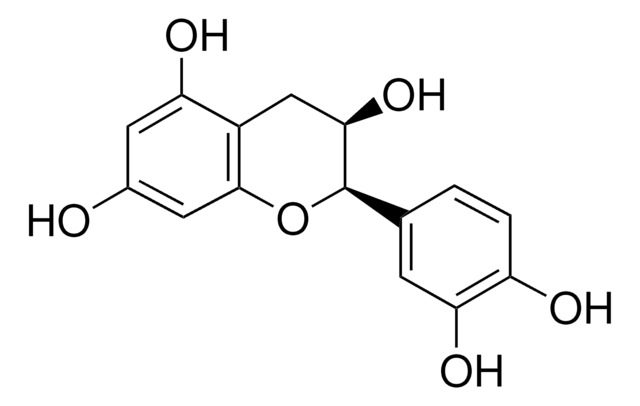
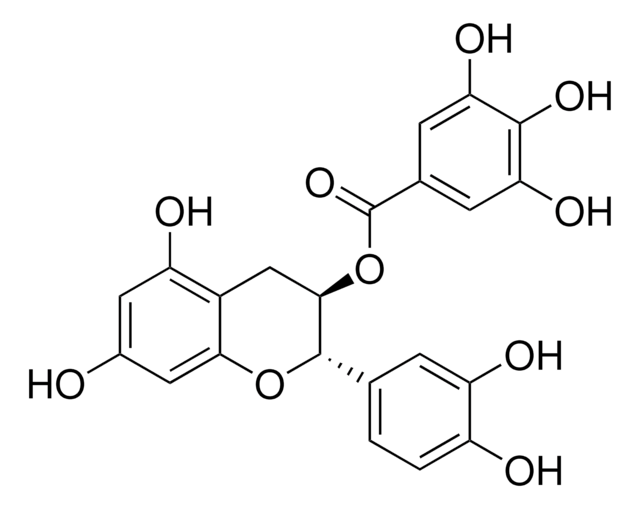
(−)-Epigallocatechin gallate
≥95%
Synonym(s):
(−)-cis-2-(3,4,5-Trihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate, (−)-cis-3,3′,4′,5,5′,7-Hexahydroxy-flavane-3-gallate, EGCG
About This Item
Recommended Products
Quality Level
assay
≥95%
solubility
H2O: ≥5 mg/mL, clear
application(s)
metabolomics
vitamins, nutraceuticals, and natural products
storage temp.
2-8°C
SMILES string
Oc1cc(O)c2C[C@@H](OC(=O)c3cc(O)c(O)c(O)c3)[C@H](Oc2c1)c4cc(O)c(O)c(O)c4
InChI
1S/C22H18O11/c23-10-5-12(24)11-7-18(33-22(31)9-3-15(27)20(30)16(28)4-9)21(32-17(11)6-10)8-1-13(25)19(29)14(26)2-8/h1-6,18,21,23-30H,7H2/t18-,21-/m1/s1
InChI key
WMBWREPUVVBILR-WIYYLYMNSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- as an anti-tumor agent on murine TRAMP metastatic prostate cell line by cell proliferation assay, apoptosis detection and modified Boyden-chamber assay
- induces cell death in acute myeloid leukaemia through death associated protein kinase-2 pathway analyzed through cell viability, cell cycle and apoptosis assay
- as an inhibitor of osteoclast differentiation in murine preosteoclast cell line RAW264.7
- to promote myogenic differentiation
Biochem/physiol Actions
signalword
Warning
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Irrit. 2 - Skin Sens. 1
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Chronic inflammation is an underlying factor in the development and progression of many of the chronic diseases of aging, such as arthritis, atherosclerosis, diabetes, and cancer.
Sigma-Aldrich presents an article about how proliferatively active cells require both a source of carbon and of nitrogen for the synthesis of macromolecules. Although a large proportion of tumor cells utilize aerobic glycolysis and shunt metabolites away from mitochondrial oxidative phosphorylation, many tumor cells exhibit increased mitochondrial activity.
Information on fatty acid synthesis and metabolism in cancer cells. Learn how proliferatively active cells require fatty acids for functions such as membrane generation, protein modification, and bioenergetic requirements. These fatty acids are derived either from dietary sources or are synthesized by the cell.
Cancer research has revealed that the classical model of carcinogenesis, a three step process consisting of initiation, promotion, and progression, is not complete.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service