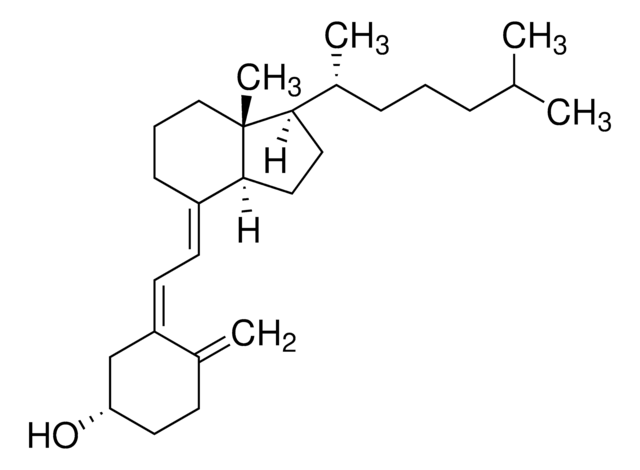
E5750
Ergocalciferol
40,000,000 USP units/g
Synonym(s):
Calciferol, Ercalciol, Ergosterol irradiated, Irradiated ergosterol, Vitamin D2
About This Item
Recommended Products
biological source
synthetic (organic)
Quality Level
assay
≥97%
form
powder
specific activity
40,000,000 USP units/g
technique(s)
HPLC: suitable
color
white to off-white
mp
114-118 °C (lit.)
storage temp.
2-8°C
SMILES string
CC(C)[C@@H](C)/C=C/[C@@H](C)[C@@]1([H])CC[C@@]([C@]1(C)CCC/2)([H])C2=C\C=C(C[C@@H](O)CC3)/C3=C
InChI
1S/C28H44O/c1-19(2)20(3)9-10-22(5)26-15-16-27-23(8-7-17-28(26,27)6)12-13-24-18-25(29)14-11-21(24)4/h9-10,12-13,19-20,22,25-27,29H,4,7-8,11,14-18H2,1-3,5-6H3/b10-9+,23-12+,24-13-/t20-,22+,25-,26+,27-,28+/m0/s1
InChI key
MECHNRXZTMCUDQ-RKHKHRCZSA-N
Gene Information
human ... VDR(7421)
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
Packaging
Quantity
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - STOT RE 1 Oral
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Vitamin D2 (ergocalciferol) is naturally synthesized from ergosterol by invertebrates, fungi, and plants in response to ultraviolet B irradiation, while vitamin D3 synthesis (cholecalciferol) is uniquely initiated in the skin of vertebrates. During sun exposure, ultraviolet B photons are absorbed by 7-dehydrocholesterol, which is found within the plasma membranes of epidermal and dermal skin layers. This reaction yields an unstable derivative of 7-dehydrocholesterol, named precholecalcitrol, which rapidly rearranges to vitamin D3. Vitamin D binding protein (DBP) is a carrier protein responsible for drawing vitamin D3 from the plasma membrane into the dermal capillaries within the extracellular space.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service