G5170
Galectin-3 human
recombinant, expressed in E. coli, lyophilized powder
Synonym(s):
CBP 35, Carbohydrate-binding protein 35, Gal-3, Galactose-specific lectin 3, Galactoside-binding protein
Sign Into View Organizational & Contract Pricing
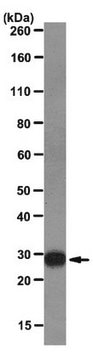
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in E. coli
Quality Level
form
lyophilized powder
UniProt accession no.
storage temp.
−20°C
Gene Information
human ... LGALS3(3958)
Related Categories
General description
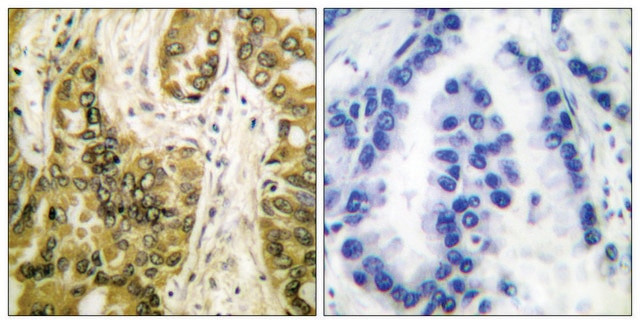
Galectin-3 protein comprises a N-terminal flexible domain and a C-terminal carbohydrate-recognition domain (CRD). It is mapped to human chromosome 14q22.3. Galectin-3 is expressed in sensory neurons, immune endothelial and epithelial cells.
Application
Galectin-3 human has been used:
- to test its interaction with N-acetyl lactosamine coated onto quantum dots
- to optimize Gal3-induced hemagglutination measurements in non-agglutinated or agglutinated chicken red blood cells (RBCs)
- in Gal-3 binding assay of serum samples from multiple sclerosis patients
Biochem/physiol Actions
Galectin-3 (Gal3) has anti-apoptotic property and mediates adhesion of cancer cells to endothelium. The activity of Gal3 is inhibited by lactose. High levels of Gal3 is associated with cardiovascular disease and is a potential biomarker in fibrosis and inflammation associated with heart failure. Gal3 is involved in variety of biological events from differentiation to host defense and immunomodulation. Gal3 gene deletion is correlated to renal function anomalies like nephropathy. It is implicated in the pathogenesis of retinopathy and non-alcoholic fatty liver disease (NAFLD).
Galectin-3 has been associated with the inhibition of apoptosis and the progression of cancer, as well as being a mediator of inflammation. Studies have found a positive correlation between the expression of galectin-3 and tumorigenicity and metastasis in colon, liver, and thyroid cancer.
Other Notes
Galectin-3 is a member of the family of animal lectins, which selectively binds β-galactoside residues.
Physical form
The product is lyophilized from water with 2 μg of lactose as stabilizer per μg of galectin-3.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Matthew R Kovak et al.
American journal of reproductive immunology (New York, N.Y. : 1989), 72(4), 403-412 (2014-05-28)
Galectin-3 is a β-galactoside binding protein with immunomodulatory properties and exerts its extracellular functions via interactions with glycoconjugate ligands. Therefore, to elucidate the function of galectin-3, binding ligands in human seminal plasma were investigated. Galectin-3 binding proteins were isolated from
Rui Dong et al.
International journal of molecular medicine, 41(2), 599-614 (2017-12-06)
Galectin-3 is a member of the galectin family, which are β‑galactoside‑binding lectins with ≥1 evolutionary conserved carbohydrate‑recognition domain. It binds proteins in a carbohydrate‑dependent and ‑independent manner. Galectin‑3 is predominantly located in the cytoplasm; however, it shuttles into the nucleus
Wei Zhao et al.
Analytical biochemistry, 571, 37-39 (2019-02-25)
Hemagglutination inhibition (HAI) assay is a simple method quantifying relative binding activities of glycan-lectin interactions. Currently, interpretation of HAI data remains a manual task depending on visual observation. In this study we developed a digital data reading method for HAI
Synthesis of multivalent N-acetyl lactosamine modified quantum dots for the study of carbohydrate and galectin-3 interactions
Yang Y, et al.
Tetrahedron, 68(35), 7148-7154 (2012)
A Hoverfelt et al.
Diabetologia, 53(9), 1903-1907 (2010-05-22)
The AGE receptors 1, 2 and 3, which are encoded by DDOST, PRKCSH and LGALS3, respectively, may be involved in the pathogenesis of diabetic complications. We sought to find out whether these genes are associated with diabetic nephropathy, cardiovascular disease
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service