M3935
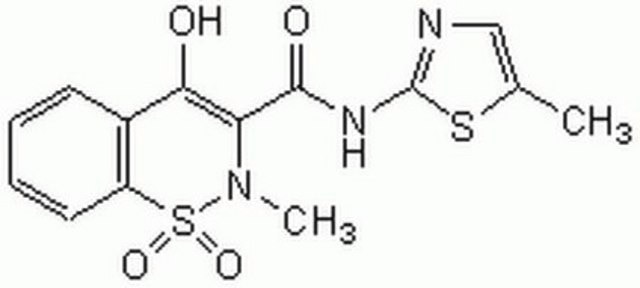
Meloxicam sodium salt hydrate
≥98% (HPLC)
Synonym(s):
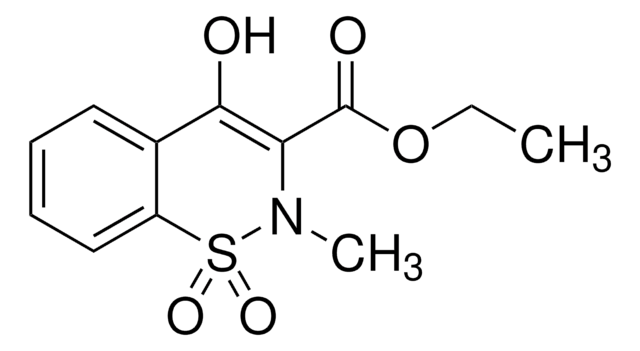
4-Hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide 1,1-dioxide sodium hydrate, Metacam sodium salt hydrate, Mobec sodium salt hydrate, UH-AC 62XX sodium salt hydrate
About This Item
Recommended Products
assay
≥98% (HPLC)
form
powder
color
light yellow to dark yellow
solubility
DMSO: 5 mg/mL, clear
originator
Boehringer Ingelheim
storage temp.
room temp
SMILES string
O.[Na+].CN1C(C(=O)Nc2ncc(C)s2)=C([O-])c3ccccc3S1(=O)=O
InChI
1S/C14H13N3O4S2.Na.H2O/c1-8-7-15-14(22-8)16-13(19)11-12(18)9-5-3-4-6-10(9)23(20,21)17(11)2;;/h3-7,18H,1-2H3,(H,15,16,19);;1H2/q;+1;/p-1
InChI key
IZBZAOZGNNQKPJ-UHFFFAOYSA-M
Gene Information
human ... PTGS2(5743)
Application
- as a cyclooxygenase-2 (COX-2) inhibitor in solid Ehrlich tumor
- as an alternative to diclofenac nonsteroidal anti-inflammatory drugs (NSAID) to treat vultures
- as a reference standard in liquid chromatography electrospray ionisation mass spectrometry (LC-ESI/MS)
- to test its effect on prostaglandin (PGE2) production in lipopolysaccharide (LPS)-induced COX-2 protein expression in RAW246.7 cells
Biochem/physiol Actions
Features and Benefits
related product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Related Content
Discover Bioactive Small Molecules for Lipid Signaling Research
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service