MSQC4
SILu™Lite SigmaMAb Universal Antibody Standard human
Synonym(s):
IgG1 light, Mass Spectrometry Universal Antibody Standard, SILu™Lite SigmaMAb Universal Antibody Standard human, recombinant IgG1 lambda light antibody, SigmaMAb
About This Item
Recommended Products
Related Categories
General description
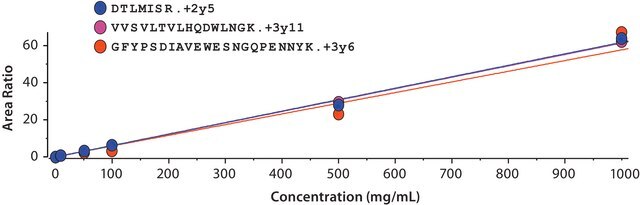
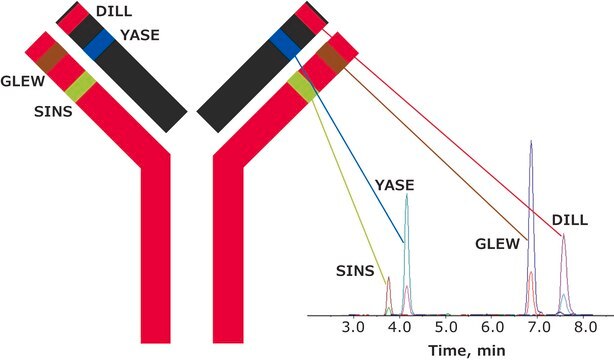
It consists of two identical heavy chains and two identical light chains. The heavy chains and light chains are linked by one disulfide bond. The heavy chains are linked by two disulfide bonds located in a hinge domain. The other 12 cysteine bonds are intramolecularly restricted to six different globular domains. The antibody sequence has been evaluated by intact mass and peptide mapping using four different enzymes: chymotrypsin, Asp-N and Glu-C endoproteinases and trypsin. Sequence coverage of 100% was obtained.
Application
Features and Benefits
Description / Composition / Modification / Average Mass (Da)
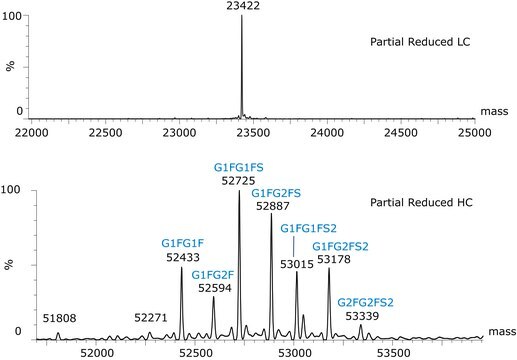
Light chain, reduced / C1006H1555N267O333S7 / Pyroglutamic acid (Q) / 22942.2
Heavy chain, reduced / C2181H3393N587O663S16 / (no modification) / 48957.8
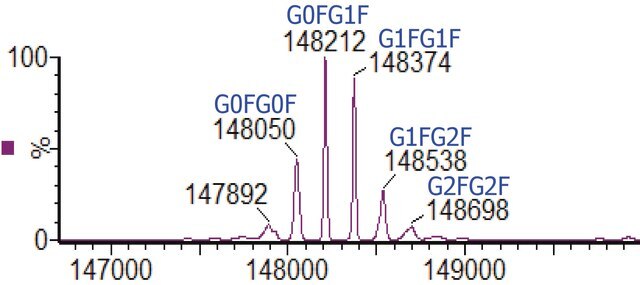
C2237H3485N591O702S16 / G0F / 50403.2
C2243H3495N591O707S16 / G1F / 50565.3
C2249H3505N591O712S16 / G2F / 50727.5
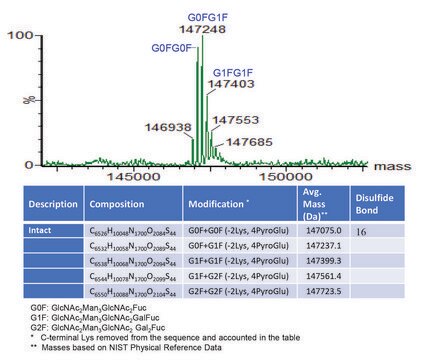
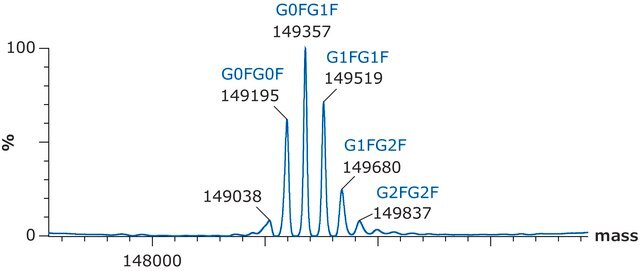
Native intact mass, non-reduced / C6374H9864N1708O1992S46 / 2 X Pyroglutamic acid (Q) / 143767.7
C6486H10048N1716O2070S46 / G0F+G0F / 146658.4
C6492H10058N1716O2075S46 / G0F+G1F / 146820.6
C6498H10068N1716O2080S46 / G1F+G1F / 146982.7
C6504H10078N1716O2085S46 / G1F+G2F / 147144.8
C6510H10088N1716O2090S46 / G2F+G2F / 147307.0
Physical form
Preparation Note
Analysis Note
EVQLVESGGGLVQPGGSLRLSCVASGFTLNNYDMHWVRQGIGKGLEWVSKI
GTAGDRYYAGSVKGRFTISRENAKDSLYLQMNSLRVGDAAVYYCARGAGRW
APLGAFDIWGQGTMVTVSS|ASTKGPSVFPLAPSSKSTSGGTAALGCLVKDYF
PEPVTVSWNSGALTSGVHTFPAVLQSSGLYSLSSVVTVPSSSLGTQTYICNVN
HKPSNTKVDKKVEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISR
TPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRV
VSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPS
RDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFL
YSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPG
SigmaMab Light Chain
QSALTQPRSVSGSPGQSVTISCTGTSSDIGGYNFVSWYQQHPGKAPKLMIY
DATKRPSGVPDRFSGSKSGNTASLTISGLQAEDEADYYCCSYAGDYTPGV
VFGGGTKLTVL|GQPKAAPSVTLFPPSSEELQANKATLVCLISDFYPGAVTV
AWKADSSPVKAGVETTTPSKQSNNKYAASSYLSLTPEQWKSHRSYSCQ
VTHEGSTVEKTVAPTECS
Other Notes
Reconstitute the contents of the vial by adding 500μL of ultrapure water or phosphate buffer, and mixing vigorously. The solubilized product can be further diluted as needed.
Legal Information
related product
supplement
Storage Class
11 - Combustible Solids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Residual presence of host cell proteins (HCPs) in recombinant therapeutic products has considerable clinical safety risks associated with a potential immunological response in patients.
Comparative analysis of different columns in resolving medium-sized fragments of monoclonal antibodies, after digestion using dithiothreitol (DTT) or IdeS (a protease), by Reversed-Phase Chromatography.
The use of PNGase Fast denaturing buffer and enzyme yielded results similar to a conventional 20-hour protocol with overnight digest while reducing workflow time to about 1 hour with a 15-minute digest.
Step-by-step workflows for the intact mass analysis, peptide mapping, and N-glycan analysis of the monoclonal antibody― adalimumab, for an accurate characterization of the critical quality attributes (CQAs) to ensure drug safety and efficacy. Read more.
Protocols
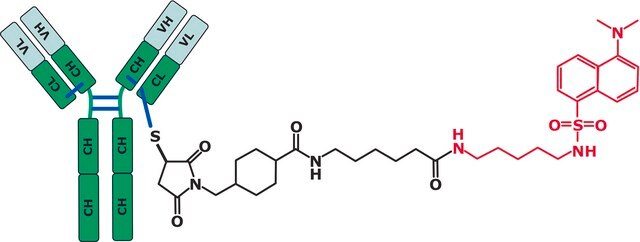
Here we show how LC and MS methods may be optimized using a non-toxic surrogate of the ADC, an “ADC-mimic”, that behaves very similarly to the Cys-linked ADC Adcetris (Seattle Genetics).
BIOshell™ IgG 1000 Å C4 UHPLC Column for Improved Biomacromolecule Separations
A complete workflow for the intact and middle-up mass analysis of reduced and non-reduced monoclonal antibodies based on SEC-MS with sample preparation by protein-A affinity clean-up.
Related Content
Step-by-step reversed phase UHPLC-MS workflow for middle-up mass analysis of an immunoglobulin G antibody, consisting of antibody purification, IdeS proteolysis and reduction, mass spectrometer calibration, mAb quantification, and a system suitability test.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service