P1868
Protein Phosphatase 2A2 from bovine kidney
buffered aqueous glycerol solution
Synonym(s):
PP2A2
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
biological source
bovine kidney
Quality Level
form
buffered aqueous glycerol solution
specific activity
~2.0 U/vial
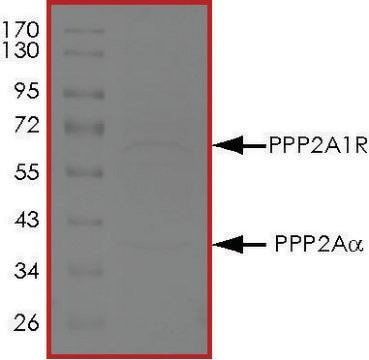
mol wt
dimer 101 kDa (65 kDa and 36 kDa)
shipped in
dry ice
storage temp.
−70°C
General description
Protein phosphatase 2A (PP2A) is a specific protamine-kinase-inactivating phosphatase, one common physiological form of which is PP2A2.
Application
Protein phosphatase 2A2 has been used in a study to investigate two heat-stable protein inhibitors. It has also been used in a study to describe the purification and properties of a protamine kinase from bovine kidney microsomes.
Biochem/physiol Actions
Protein Phosphatase 2A2 from bovine kidney was shown to be a unique inhibitor of protamine kinase while other phosphatases in the same family including PP1, PP2B, PP2C did not show any inhibition.
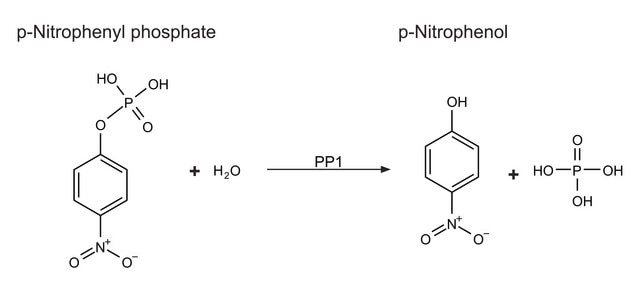
Unit Definition
One unit will release 1 nanomole of inorganic phosphate from (32)P-labeled phosphorylase per minute at pH 7.0 at 30 deg C.
Physical form
Solution of 1 μg per vial in 50 μl of 50 mM Tris-HCl, pH 7.0, containing 14 mM β-Mercaptoethanol, 1mM benzamidine, 0.1 mM PMSF, 1mM EDTA, and 50% glycerol.
signalword
Warning
hcodes
Hazard Classifications
Skin Sens. 1
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protein Phosphatase 2A is a specific protamine-kinase-inactivating phosphatase.
Amick, G.D, et al.
The Biochemical Journal, (Pt 3), 1019-1022 (1992)
G D Amick et al.
Archives of biochemistry and biophysics, 297(1), 80-85 (1992-08-15)
About an eightfold increase in protamine kinase activity was detected following extraction of highly purified microsomes from bovine kidney with 1% Triton X-100. Relative to the soluble fraction, the microsomes contained about 30% protamine kinase activity. The microsomal protamine kinase
H R Matthews et al.
FEBS letters, 364(1), 51-54 (1995-05-01)
Whole cell extracts from rat liver or spinach leaves contain divalent ion-independent protein histidine phosphatase activity due to phosphatases of the PP1/PP2A family. In the rat liver extract, almost all the activity was found in the PP1, PP2A1 and PP2A2
M Li et al.
Biochemistry, 34(6), 1988-1996 (1995-02-14)
Two heat-stable protein inhibitors of protein phosphatase 2A (PP2A), tentatively designated I1PP2A and I2PP2A, have been purified to apparent homogeneity from extracts of bovine kidney. The purified preparations of I1PP2A exhibited an apparent M(r) approximately 30,000 and 250,000 as determined
A Hiraga et al.
The Biochemical journal, 346 Pt 2, 433-439 (2000-03-24)
Protein phosphatase (PP) 2A1, a trimer composed of A-, B- and C-subunits in the PP2A family, has been regarded as a principal form localizing at microtubules (MT), but PP2A2, the dimer of A- and C-subunits, has not. Substantiating the claim
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service