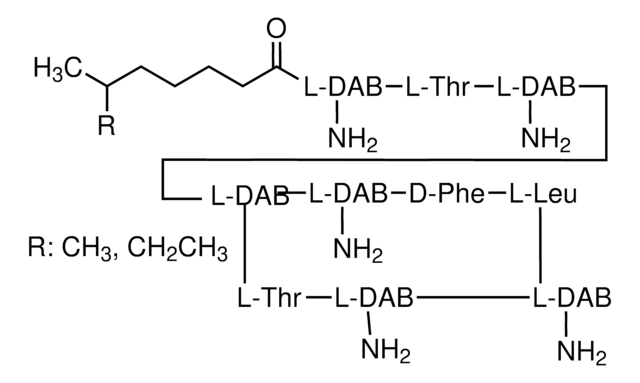
P9208
Polymyxin Acylase from Pseudomonas sp.
≥0.1 units/mg solid
Synonym(s):
Peptide N-fatty acylase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
biological source
bacterial (Pseudomonas spp.)
Quality Level
form
solid
specific activity
≥0.1 units/mg solid
relevant disease(s)
cancer
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
Application
Polymyxin acylase, from Pseudomonas sp., is a N-myristoyl cleaving enzyme that has been used to determine the N-myristoyl peptide sequence and may be useful in cancer research since it has antitumor activity against murine and human tumor cells .
Biochem/physiol Actions
Polymyxin acylase, from Pseudomonas sp. deacylates polymyxin group antibiotics and long-chain fatty acyl groups of proteins. Polymyxin acylase has an affinity for long-chain fatty acyl proteins in human carcinoma cells .
Quality
Crude actone powder;
Unit Definition
One unit will hydrolyze 1 μmole of N-octanoyl-5-aminovaleric acid per min at pH 8.0 at 37°C.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Yasuda et al.
Biological & pharmaceutical bulletin, 18(4), 615-617 (1995-04-01)
Polymyxin acylase from Pseudomonas sp. M-6-3 can deacylate not only polymyxin group antibiotics, but also the long-chain fatty acyl group of proteins and peptides. We found the in vitro antitumor activity of polymyxin acylase against murine and human tumor cells
S Misumi et al.
Biochemical and biophysical research communications, 217(2), 632-639 (1995-12-14)
Polymyxin acylase isolated from Pseudomonas sp. M-6-3 was used as an N-myristoyl cleaving enzyme in order to determine a part of the N-terminal amino acid sequence of N-myristoyl proteins. The enzyme hydrolyzed a number of N-myristoyl oligopeptides at various hydrolysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service