S5921
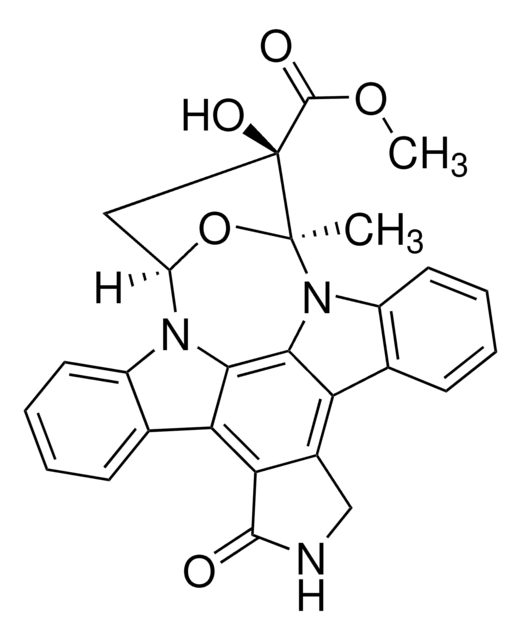
Staurosporine
from Streptomyces sp., ≥95% (HPLC), film, protein kinase inhibitor
Synonym(s):
protein kinase c inhibitor, staurosporine, Antibiotic AM-2282
About This Item
Recommended Products
product name
Staurosporine from Streptomyces sp., for molecular biology, ≥95% (HPLC)
grade
for molecular biology
Quality Level
assay
≥95% (HPLC)
antibiotic activity spectrum
fungi
mode of action
enzyme | inhibits
storage temp.
2-8°C
SMILES string
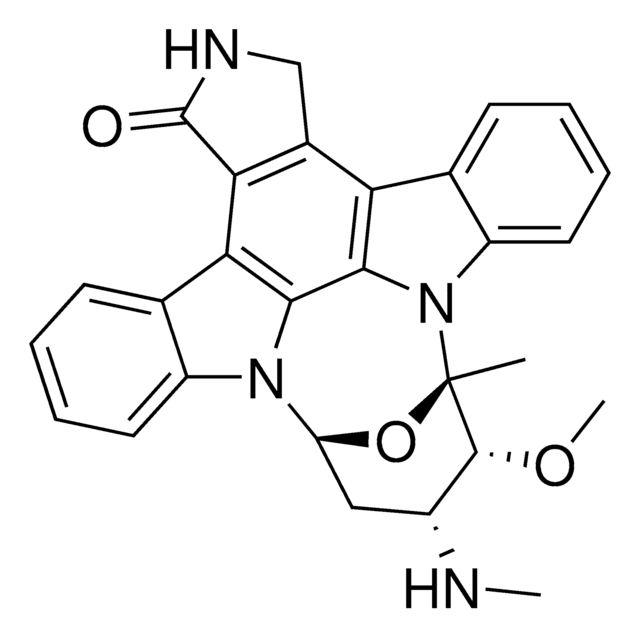
CN[C@@H]1C[C@@H]2O[C@@](C)([C@@H]1OC)n3c4ccccc4c5c6CNC(=O)c6c7c8ccccc8n2c7c35
InChI
1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20?,26-,28+/m1/s1
InChI key
HKSZLNNOFSGOKW-ZGQXJOJZSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
Biochem/physiol Actions
Features and Benefits
Analysis Note
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 4 - Carc. 1B - Muta. 1B - Repr. 2
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Protein-based drug transporters are found in most tissues including liver, kidney, intestine, and brain. These transporters are particularly important in cancer treatment and multi-drug resistance research. Understanding the specific mechanisms of tumor cell transporters is becoming an essential aspect of chemotherapeutic drug design.
Related Content
Discover Bioactive Small Molecules for ADME/Tox
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service