SML1797
L67
≥98% (HPLC)
Synonym(s):
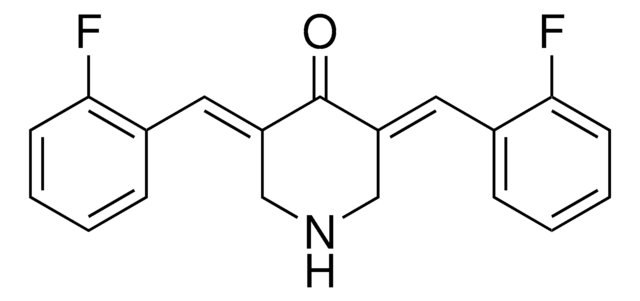
LigI/III inhibitor L67, N-(3,5-Dibromo-4-methylphenyl)-glycine 2-[(2-hydroxy-5-nitrophenyl)methylene]hydrazide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H14Br2N4O4
CAS Number:
Molecular Weight:
486.11
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
assay
≥98% (HPLC)
form
powder
color
white to beige
solubility
DMSO: 10 mg/mL, clear
storage temp.
−20°C
SMILES string
O=C(N/N=C/C1=CC([N+]([O-])=O)=CC=C1O)CNC2=CC(Br)=C(C)C(Br)=C2
Biochem/physiol Actions
L67 is a potent and specific inhibitor of DNA ligase IIIα (LigIIIα) that preferentially targets mitochondrial LigIIIα resulting in mitochondrial dysfunction. L67 preferentially targets cancer cell mitochondria resulting in enhanced ROS production and caspase 1-dependent apoptosis. L67 in combination with PARP inhibitors decreases survival rate of therapy resistant breast cancer and leukemia cells.
hcodes
pcodes
Hazard Classifications
Aquatic Chronic 4
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Annahita Sallmyr et al.
Cancer research, 76(18), 5431-5441 (2016-08-10)
Elevated levels of DNA ligase IIIα (LigIIIα) have been identified as a biomarker of an alteration in DNA repair in cancer cells that confers hypersensitivity to a LigIIIα inhibitor, L67, in combination with a poly (ADP-ribose) polymerase inhibitor. Because LigIIIα
L A Tobin et al.
Oncogene, 32(14), 1784-1793 (2012-05-30)
Resistance to imatinib (IM) and other tyrosine kinase inhibitors (TKI)s is an increasing problem in leukemias caused by expression of BCR-ABL1. As chronic myeloid leukemia (CML) cell lines expressing BCR-ABL1 utilize an alternative non-homologous end-joining pathway (ALT NHEJ) to repair
Xi Chen et al.
Cancer research, 68(9), 3169-3177 (2008-05-03)
Based on the crystal structure of human DNA ligase I complexed with nicked DNA, computer-aided drug design was used to identify compounds in a database of 1.5 million commercially available low molecular weight chemicals that were predicted to bind to
Rajeswari Jayavaradhan et al.
Journal of molecular biology, 431(1), 102-110 (2018-05-12)
The efficient site-specific DNA double-strand breaks (DSB) created by CRISPR/Cas9 has revolutionized genome engineering and has great potential for editing hematopoietic stem/progenitor cells (HSPCs). However, detailed understanding of the variables that influence choice of DNA-DSB repair (DDR) pathways by HSPC
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service