SRP0116
Sirtuin 2 human
recombinant, expressed in E. coli, ≥80% (SDS-PAGE)
Synonym(s):
SIR2L2, SIRT2, sir2-like 2
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352200
NACRES:
NA.32
Recommended Products
biological source
human
recombinant
expressed in E. coli
assay
≥80% (SDS-PAGE)
form
aqueous solution
mol wt
35.5 kDa
packaging
pkg of 100 μg
storage condition
avoid repeated freeze/thaw cycles
concentration
>0.02 mg/mL
NCBI accession no.
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... SIRT2(22933)
General description
Silent information regulator 2 (Sir2) or sirtuin 2 is encoded by the gene mapped to human chromosome 19q13.2. It belongs to class Ib of sirtuin protein family. Sir2 is characterized with a catalytic domain containing two different domains that bind NAD and the acetyl-lysine substrate, respectively. Apart from this, it also constitutes variable NH2 and COOH-terminal domains involved in the regulation of subcellular localizations and catalytic activity. sirtuin 2 is mainly expressed in cytosol but its migration between cytosol and nucleus facilitates the deacetylation of both α-tubulin and histones.
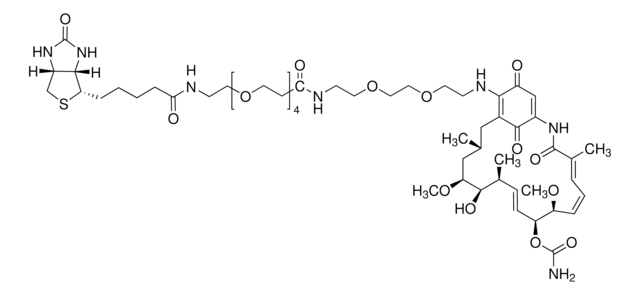
Human Sirtuin 2, GenBank Accession No. NM_030593, amino acids 50-356 with C-terminal His tag, MW = 35.5kDa, expressed in Escherichia coli expression system.
Human Sirtuin 2, GenBank Accession No. NM_030593, amino acids 50-356 with C-terminal His tag, MW = 35.5kDa, expressed in Escherichia coli expression system.
Application
Useful for the study of enzyme kinetics, screening inhibitors, and selectivity profiling.
Biochem/physiol Actions
Silent information regulator 2 (Sir2) acts as a nicotine adenine dinucleotide (NAD) dependent deacetylase and is implicated in various biological processes such as regulation of transcriptional repression, recombination, the cell-division cycle, microtubule organization, and cellular responses to DNA-damaging agents. In addition, sirtuins also regulate the process involved in aging. Sir2 plays a vital role in mammalian metabolic homeostasis and is considered to be a potent therapeutic target for the treatment of type 2 diabetes. Sir2 has a crucial role in reduction of microglial activation and brain inflammation. Therefore, loss of Sir2 activity increases the risk of susceptibility to neurodegenerative diseases.
Unit Definition
One unit is defined as the amount of enzyme required to deacetylate 1 pmol of substrate/min at 37°C.
Physical form
Formulated in 25 mM Tris-HCl, pH 8.0, 100 mM NaCl, 0.05% Tween-20, 50% glycerol and 3 mM DTT.
Preparation Note
Thaw on ice. Upon first thaw, briefly spin tube containing enzyme to recover full content of the tube. Aliquot enzyme into single use aliquots. Store remaining undiluted enzyme in aliquots at -70°C. Note: Enzyme is very sensitive to freeze/thaw cycles.
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation.
Pais TF
The Embo Journal, 32(19), 2603-2616 (2013)
Yi Shi et al.
eLife, 3, e02349-e02349 (2014-06-19)
Recent studies suggested an essential role for seryl-tRNA synthetase (SerRS) in vascular development. This role is specific to SerRS among all tRNA synthetases and is independent of its well-known aminoacylation function in protein synthesis. A unique nucleus-directing domain, added at
Emerging Role of Sirtuin 2 in the Regulation of Mammalian Metabolism.
Gomes P
Trends in Pharmacological Sciences, 36(11), 756-768 (2015)
Ruwin Pandithage et al.
The Journal of cell biology, 180(5), 915-929 (2008-03-12)
Cyclin-dependent kinases (Cdks) fulfill key functions in many cellular processes, including cell cycle progression and cytoskeletal dynamics. A limited number of Cdk substrates have been identified with few demonstrated to be regulated by Cdk-dependent phosphorylation. We identify on protein expression
Sirtuins: Sir2-related NAD-dependent protein deacetylases.
North BJ and Verdin E.
Genome Biology, 5(5), 224-224 (2004)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service