SRP0146
PRMT5/MEP50 Active human
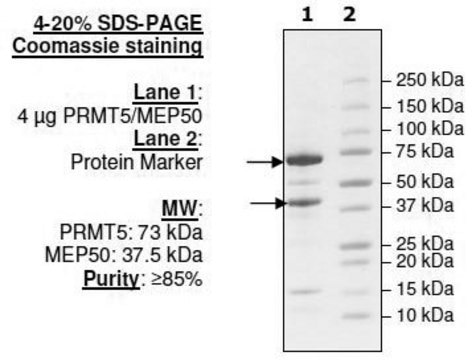
recombinant, expressed in baculovirus infected insect cells, ≥70% (SDS-PAGE)
Synonym(s):
Arginine methyltransferase 5, HMT1 hnRNP methyltransferase-like 5(HRMT1L5), ICln-binding protein (IBP72) 72 kDa, Jak-binding protein 1 (JBP1), shk1 kinase-binding protein 1 homolog (SKB1)
About This Item
Recommended Products
biological source
human
recombinant
expressed in baculovirus infected insect cells
assay
≥70% (SDS-PAGE)
form
aqueous solution
mol wt
73 kDa
packaging
pkg of 20 μg
storage condition
avoid repeated freeze/thaw cycles
concentration
>0.02 mg/mL
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... PRMT5(10419)
General description
Human PRMT5 (GenBank Accession No. NM_006109), amino acids 2-end, with N-terminal DDDDK tag (FLAG), MW = 73kDa, expressed in a Baculovirus infected Sf9 cell expression system.
Application
Biochem/physiol Actions
Unit Definition
Physical form
Preparation Note
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service