T8512
Activated Thiol–Sepharose™ 4B
lyophilized powder
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Recommended Products
form
lyophilized powder
Quality Level
extent of labeling
1 μmol per mL
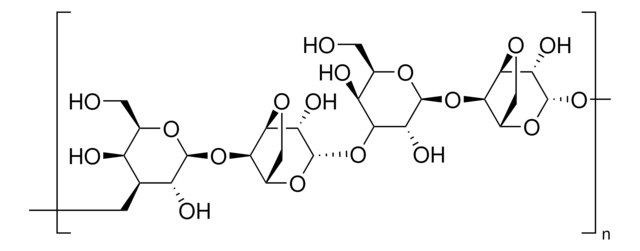
matrix
Sepharose 4B
matrix activation
cyanogen bromide
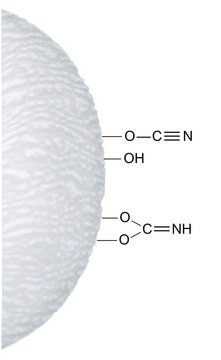
matrix active group
glutathione 2-pyridyl disulfide
matrix attachment
N-terminal amino group
matrix spacer
10 atoms (when ligands are coupled through the disulfide groups)
swelling
1 g swells to 4-5 mL
storage temp.
2-8°C
Application
Activated thiol Sepharose™ 4B is used in protein chromatography, affinity chromatography and activated/functionalized matrices. Activated thiol Sepharose™ 4B has been used to provide the first report of the isolation of aminopeptidase H from a reptile. Activated thiol Sepharose™ 4B has also been used to purify and characterize a neuropeptide-inactivating peptidase.
Physical form
Lyophilized powder stabilized with lactose and dextran
Legal Information
Sepharose is a trademark of Cytiva
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
N Agell et al.
The Biochemical journal, 273 ( Pt 3), 615-620 (1991-02-01)
A ubiquitin hydrolase that removes ubiquitin from a multi-ubiquitinated protein has been purified 600-fold from Saccharomyces cerevisiae. Four different ubiquitin-protein conjugates were assayed as substrates during the purification procedure. Enzymic activities that removed ubiquitin from ubiquitinated histone H2A, a ubiquitin-ubiquitin
Abdullah Ozer et al.
Nucleic acids research, 41(14), 7167-7175 (2013-06-06)
The non-specific binding of undesired ligands to a target is the primary factor limiting the enrichment of tight-binding ligands in affinity selection. Solution-phase non-specific affinity is determined by the free-energy of ligand binding to a single target. However, the solid-phase
N Iwatsuki et al.
Biochemistry, 19(6), 1172-1176 (1980-03-18)
DNA photolyase purified from baker's yeast by affinity chromatography on UV-irradiated DNA noncovalently bound to cellulose and by chromatography on activated thiol-Sepharose 4B yields a single protein band having a molecular weight of 51 000 when analyzed by sodium dodecyl
S Al-Jassabi
Biochemistry. Biokhimiia, 64(2), 217-222 (1999-04-03)
Aminopeptidase H was isolated and purified from fresh skeletal muscle of the lizard Agama stellio stellio by ammonium sulfate fractionation and successive chromatographies on DEAE-cellulose, Ultrogel AcA-34, activated thiol-Sepharose 4B, phenyl-Sepharose CL-4B, and DEAE-cellulose again. This is the first report
G Oshima et al.
Biological & pharmaceutical bulletin, 23(5), 532-536 (2000-05-24)
Glutathione peroxidase (GPx) activity was detected in the ascite fluid of rats injected intraperitoneally with 2.5% heat-denatured casein solution. Activity in the ascite fluid increased with time after the injection of casein, and reached a maximum at 24 h. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service