75033AST
Astec® CHIRALDEX™ G-BP Capillary GC Column
L × I.D. 30 m × 0.25 mm, df 0.12 μm
About This Item
Recommended Products
material
fused silica
Quality Level
description
GC capillary column
parameter
-10-200 °C temperature (isothermal)
-10-220 °C temperature (programmed)
Beta value
500
df
0.12 μm
technique(s)
gas chromatography (GC): suitable
L × I.D.
30 m × 0.25 mm
matrix active group
non-bonded; 2,6-di-O-pentyl-3-butyryl derivative of γ-cyclodextrin phase
application(s)
agriculture
chemicals and industrial polymers
cleaning products
clinical
cosmetics
environmental
flavors and fragrances
food and beverages
forensics and toxicology
life science and biopharma
personal care
pharmaceutical (small molecule)
column type
capillary chiral
separation technique
chiral
Looking for similar products? Visit Product Comparison Guide
Related Categories
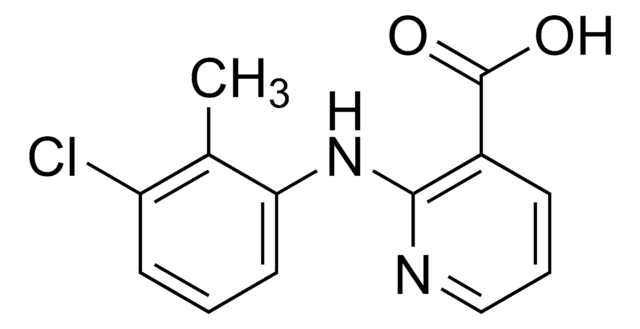
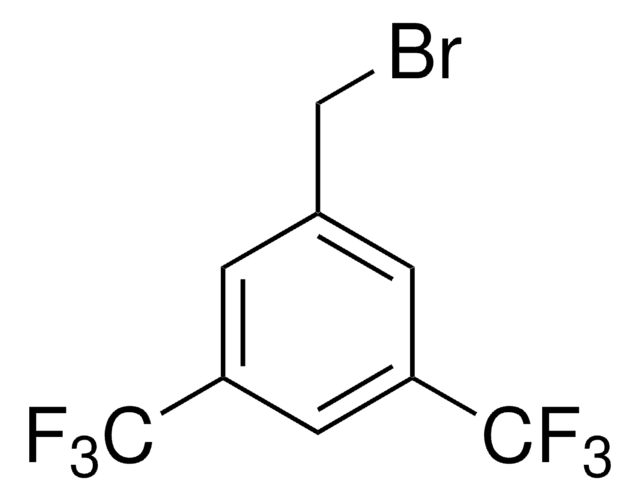
General description
Chem/Phys Resistance
- -10 °C to 200 °C isothermal, 220 °C programmed
Other Notes
Legal Information
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service