PHL80152
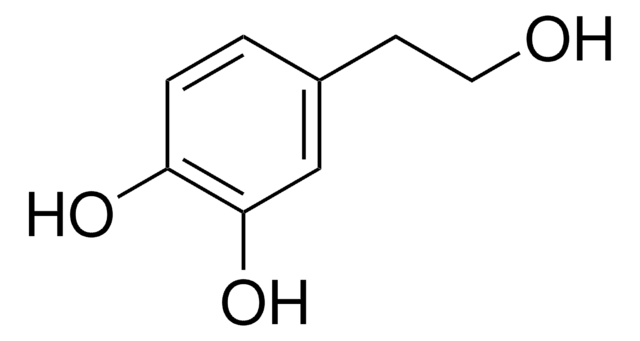
Hydroxytyrosol
phyproof® Reference Substance
Synonym(s):
3-Hydroxytyrosol, 2-(3,4-Dihydroxyphenyl)ethanol, 3,4-Dihydroxyphenethyl alcohol, DOPET, Homoprotocatechuyl alcohol
About This Item
Recommended Products
grade
primary reference standard
product line
phyproof® Reference Substance
assay
≥90.0% (HPLC)
manufacturer/tradename
PhytoLab
application(s)
food and beverages
format
neat
storage temp.
−20°C
SMILES string
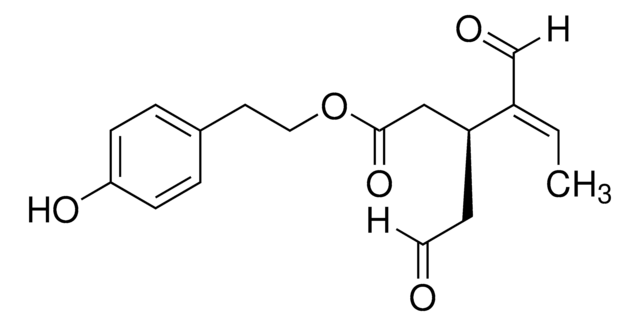
OCCc1ccc(O)c(O)c1
InChI
1S/C8H10O3/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,9-11H,3-4H2
InChI key
JUUBCHWRXWPFFH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Neuroprotective Applications: Research highlights Hydroxytyrosol′s neuroprotective properties, particularly in treating neuroblastoma. This study assesses the therapeutic potential of olive oil components, including Hydroxytyrosol, underscoring their role in neurodegenerative disease studies (Goncalves et al., 2024).
- Antioxidant Properties: Hydroxytyrosol is recognized for its significant antioxidant effects. This study focuses on the metabolic changes in olives and elucidates Hydroxytyrosol′s contribution, reinforcing its importance in antioxidant properties research (Miho et al., 2024).
- Anti-inflammatory Effects: The anti-inflammatory effects of Hydroxytyrosol are explored in various contexts, including its role in inhibiting chronic inflammation pathways, which is pivotal for pharmaceutical research on natural phenolic compounds in cancer and cardiovascular health (Luo et al., 2024).
Biochem/physiol Actions
Legal Information
signalword
Warning
hcodes
pcodes
Hazard Classifications
Acute Tox. 4 Oral
Storage Class
10 - Combustible liquids
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service