Y0002262
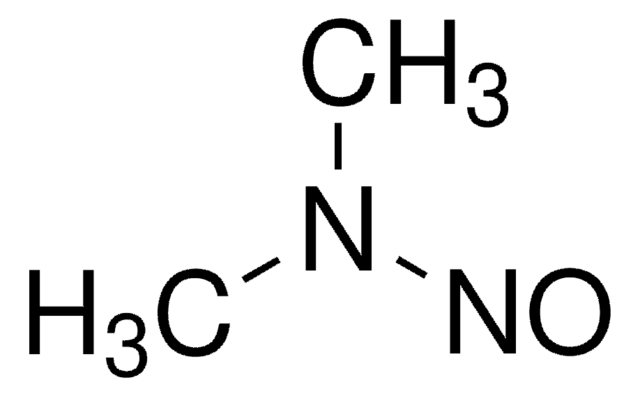
N-Nitroso-ethyl-isopropylamine
European Pharmacopoeia (EP) Reference Standard
Synonym(s):
N-Ethyl-N-nitroso-2-propanamine solution
About This Item
Recommended Products
manufacturer/tradename
EDQM
concentration
in methanol
application(s)
pharmaceutical
storage temp.
2-8°C
InChI
1S/C5H12N2O/c1-4-7(6-8)5(2)3/h5H,4H2,1-3H3
InChI key
VGGZTNNNXAUZLB-UHFFFAOYSA-N
General description
Application
It is also used to prepare N-Nitrosamines spiking solution to detect the concentration of N-nitrosamines in active substances according to the monograph 20542 of European Pharmacopoeia.
Packaging
Other Notes
signalword
Danger
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Carc. 1B - Flam. Liq. 2 - STOT SE 1
target_organs
Eyes,Central nervous system
wgk_germany
WGK 3
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Learn about LC-MS/MS method development to quantify NDMA impurity in valsartan drug substance using Titan™ C18 column based UHPLC separation
Nitrosamines have been discovered as a serious contaminant group in active pharmaceutical ingredients (API) belonging to the sartan family. This article describes a GC-MS method for the determination of nitrosamines in Valsartan tablets according to US FDA guide lines that can be used for pharma QC.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service