1173508
USP
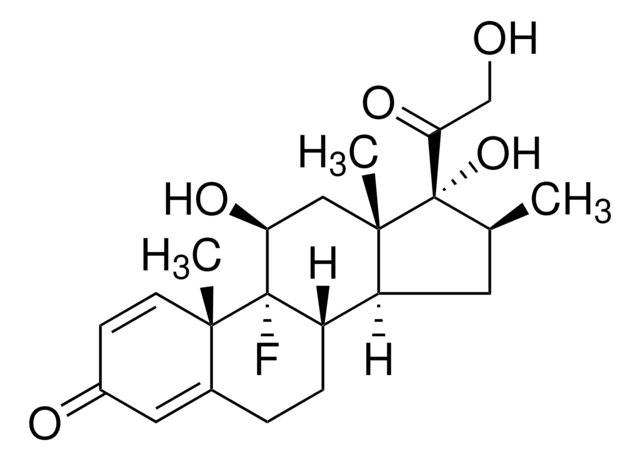
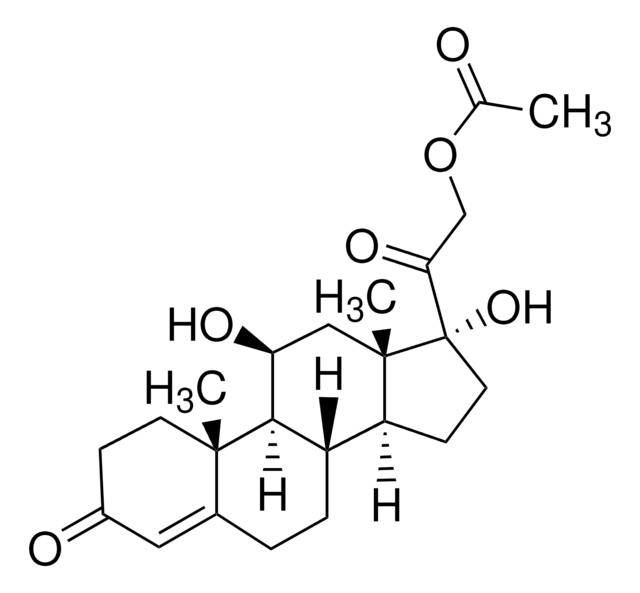
Desoximetasone
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
9-Fluoro-11β,21-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione
About This Item
Recommended Products
biological source
synthetic
agency
USP
vapor pressure
<0.0000001 kPa ( 25 °C)
API family
desoximetasone
form
powder
packaging
pkg of 200 mg
manufacturer/tradename
USP
storage condition
protect from light
color
white
solubility
acetone: freely soluble
alcohol: freely soluble
benzene: slightly soluble
chloroform: freely soluble
ether: slightly soluble
water: insoluble
application(s)
pharmaceutical (small molecule)
SMILES string
[H][C@@]12C[C@@H](C)[C@H](C(=O)CO)[C@@]1(C)C[C@H](O)[C@@]3(F)[C@@]2([H])CCC4=CC(=O)C=C[C@]34C
InChI
1S/C22H29FO4/c1-12-8-16-15-5-4-13-9-14(25)6-7-21(13,3)22(15,23)18(27)10-20(16,2)19(12)17(26)11-24/h6-7,9,12,15-16,18-19,24,27H,4-5,8,10-11H2,1-3H3/t12-,15+,16+,18+,19-,20+,21+,22+/m1/s1
InChI key
VWVSBHGCDBMOOT-IIEHVVJPSA-N
Gene Information
human ... NR3C1(2908)
Looking for similar products? Visit Product Comparison Guide
General description
Application
Analysis Note
Other Notes
related product
signalword
Warning
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Protocols
A simple, precise and sensitive Reverse-Phase High Pressure Liquid Chromatography gradient method was adapted for traceability, homogeneity and total chromatographic analysis of Dexamethasone. The given experimental conditions follow the USP43-NF38 monograph method for Dexamethasone Assay and Organic Impurity Profiling. Dexamethasone, Betamethasone, Dexamethasone acetate and Desoximetasone were baseline resolved within 20 minutes using a Titan C18 UHPLC column (2.1 x 100 mm, 1.9 µm).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service