1225317
USP
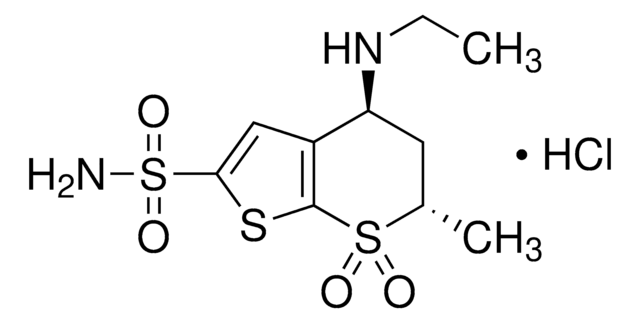
Dorzolamide Related Compound B
United States Pharmacopeia (USP) Reference Standard
Synonym(s):
rac-cis Dorzolamide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
grade
pharmaceutical primary standard
manufacturer/tradename
USP
availability
not available in (Sales restrictions may apply)
application(s)
pharmaceutical
format
neat
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including MSDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Dorzolamide Related Compound B USP reference standard for use in specified quality tests and assays.
Also used to prepare standard solutions for assay and impurity analysis using liquid chromatography method and UV detector according to the given below monographs of United States Pharmacopeia (USP):
Also used to prepare standard solutions for assay and impurity analysis using liquid chromatography method and UV detector according to the given below monographs of United States Pharmacopeia (USP):
- Dorzolamide Hydrochloride Ophthalmic Solution
- Dorzolamide Hydrochloride and Timolol Maleate Ophthalmic Solution
Analysis Note
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
Other Notes
USP issued SDS can be found here.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dorzolamide Hydrochloride Ophthalmic Solution
United States Pharmacopeia and National Formulary
United States Pharmacopeia, 46(4) (2021)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service