Identification, Assay and Organic Impurity Profiling Methods for Aripiprazole following the United States Pharmacopeia Monograph
Sonal Shinde
MilliporeSigma, Life Science Application Centre (GAC), Mumbai, India
Aripiprazole USP Monograph Methods Overview
Aripiprazole is an atypical antipsychotic and a partial dopamine agonist. It is primarily used in the treatment of schizophrenia, bipolar disorder, major depressive disorder, tic disorders, and irritability associated with autism. Aripiprazole was first approved by the U.S. Food and DrugAdministration in November 2002 for schizophrenia and by the European Medicines Agency in June 2004 for acute manic and mixed episodes associated with bipolar disorder.
Common commercial brand Names:
Abilify and Aripiprex
Aripiprazole was developed by Otsuka in Japan. In the United States, Otsuka America markets it jointly with Bristol-Myers Squibb. In 2010, sales were $4.6 billion globally; the patent expired in 2015.
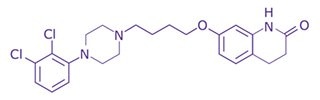
Figure 1.Chemical structure of Aripiprazole
In this compilation, we have used the USP 40–NF 35 experimental conditions for Aripiprazole in the following areas:
- Identification — FTIR
- Assay — HPLC (gradient method)
- Related Substances — HPLC (gradient method)
The HPLC methods are gradient methods, thus they are nonscalable.
The same chromatographic conditions are used for methods in both the assay and related substances, and a full validation protocol can be found using these USP Reference Standards: USP Aripiprazole RS and USP Aripiprazole Related Compound F RS.
Identification and Assay
Definition
Aripiprazole contains not less than (NLT) 98.0% and not more than (NMT) 102.0% of aripiprazole (C23H27Cl2N3O2), calculated on the dried basis.
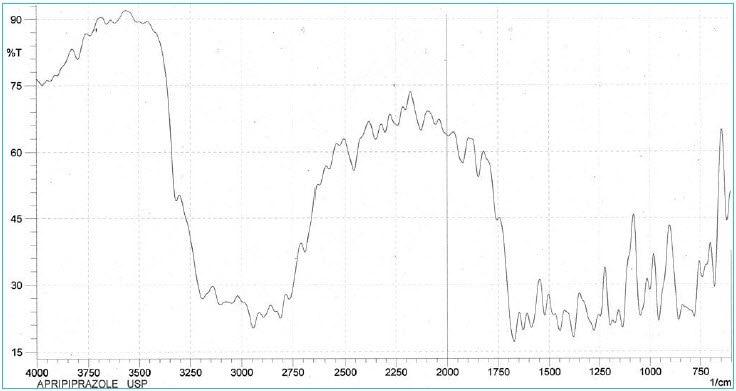
Identification—FTIR <197K>
- Infrared absorption
- The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY—HPLC (gradient method)
Procedure (protect the solutions from light):
- Diluent: Acetonitrile, methanol, water, and acetic acid (30:10:60:1)
- Solution A: Acetonitrile and 0.05% trifluoroacetic acid (10:90)
- Solution B: Acetonitrile and 0.05% trifluoroacetic acid (90:10)
- Gradient: (Table 1)
- Detector: UV 254 nm
- Column: 4.6 mm × 10 cm (3 µm) packing L1
- Flow rate: 1.2 mL/min
- Injection volume: 20 µL
Note: The gradient was established on an HPLC system with a dwell volume of approximately 650 µL.
Assay Solutions and Analysis
- System suitability solution: 1 µg/mL each of USP Aripiprazole and USP Aripiprazole Related Compound F in Diluent
- Standard solution: 0.1 mg/mL of USP Aripiprazole in Diluent
- Sample solution: 0.1 mg/mL of aripiprazole in Diluent
- System Suitability
- Samples: System suitability solution and Standard solution
Note: The relative retention times (RRT) for aripiprazole and aripiprazole related compound F are 1.0 and 1.1, respectively.
Suitability Requirements
- Resolution: NLT 2.0 between aripiprazole and aripiprazole related compound F, System suitability solution
- Tailing factor: NMT 1.5 for aripiprazole, System suitability solution
- Relative standard deviation: NMT 1.0%, Standard solution
Analysis
- Samples: Standard solution and Sample solution
- Calculate the percentage of aripiprazole (C23H27Cl2N3O2) in the portion of the sample taken.
- Result = (rU/rS) × (CS/CU) × 100
rU = peak area from the Sample solution
rS = peak area from the Standard solution
CS = concentration of USP Aripiprazole in the Standard solution (mg/mL)
CU = concentration of aripiprazole in the Sample solution (mg/mL) - Acceptance criteria: 98.0%–102.0% on the dried basis
Impurities Analysis
- Inorganic Impurities
- Residue on Ignition—USP General Chapter 281: NMT 0.1%
- Heavy Metals, Method II—USP General Chapter 2311: NMT 10 ppm
- Organic Impurities
(Protect the solutions from light.) Diluent, Solution A, Solution B, Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability: Proceed as directed in the Assay.
Analysis
- Samples: Sample solution
Calculate the percentage of each impurity in the portion of aripiprazole taken. - Result = (ri/rU) × (1/F) × 100
ri = peak response of each impurity from the Sample solution
rU = peak response of aripiprazole from the Sample solution
F = relative response factor (Table 2)
USP Reference Standards - USP Aripiprazole
- USP Aripiprazole Related Compound F
1Chapter valid until Jan. 1, 2018.
27-{4-[4-(2,3-Dichlorophenyl)piperazin-1-yl]butoxy}quinolin-2(1H)-one
34-(2,3-Dichlorophenyl)-1-[4-(2-oxo-1,2,3,4-tetrahydroquinolin-7-yloxy)butyl]piperazin 1-oxide
4For system suitability and identification purposes only
51,1¢-(Ethane-1,1-diyl)bis(2,3-dichloro-4-{4-[3,4-dihydroquinolin-2(1H)-one-7-yloxybutyl]piperazin-1-yl}benzene)
Identification Data
Infrared Absorption
The reference <197K> in a monograph signifies that the substance under examination is mixed intimately with potassium bromide. We recommend potassium bromide for IR spectroscopy—Uvasol® (Product No. 1.04907).
Assay and Related Substances Data
Chromatographic Conditions
Column: Purospher® STAR RP-18 endcapped (3 µm) 100 x 4.6 mm (Product No. 1.50469)
Injection: 20 µL
Detection: UV 254 nm
Cell: 10 µL
Flow Rate: 1.2 mL/min
Mobile Phase A: Acetonitrile and 0.05% trifluoroacetic acid (10:90 v/v)
Mobile Phase B: Acetonitrile and 0.05% trifluoroacetic acid (90:10 v/v)
Gradient: See table.
Temperature: 40 °C
Diluent: Acetonitrile, methanol, water, and acetic acid (30:10:60:1 v/v)
Sample: 1 µg/mL (1 ppm) each of aripiprazole and aripiprazole related compound F in Diluent
Pressure drop: 95–170 Bar (1,377–2,465 psi)
Suitability Requirements
- Resolution: NLT 2.0 between aripiprazole and aripiprazole related compound F
- Tailing Factor: NMT 1.5 for aripiprazole
- Relative Retention Time (RRT):
1.0 for aripiprazole and
1.1 for aripiprazole related compound F
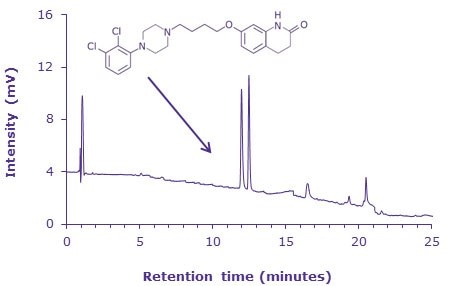
Chromatographic Data (System Suitability Solution) |
|---|
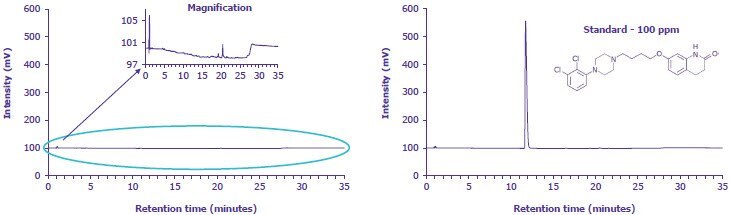
Assay and Related Substances Validation Data
A. Specificity |
|---|
B. Repeatability |
|---|
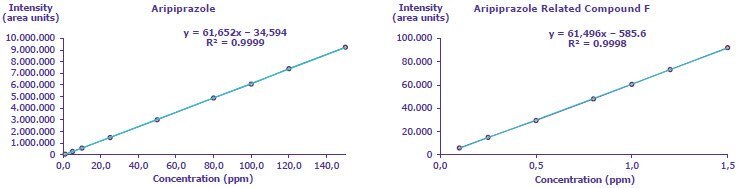
C. Linearity, Limit of Detection (LOD), and Limit of Quantitation (LOQ)
Determined by injecting seven concentration levels from 0.1 to 1.5 ppm of USP Aripiprazole Related Compound F and nine concentration levels ranging from 1.0 to 150.0 ppm of aripiprazole.
D. Linearity, LOD, LOQ (cont.)
Determined by injecting seven concentration levels from 0.1 to 1.5 ppm of USP Aripiprazole Related Compound F and nine concentration levels ranging from 1.0 to 150.0 ppm of aripiprazole.
D. LOQ Accuracy |
|---|
Recommended Products
- Acetic acid (glacial) 100% anhydrous for analysis—EMSURE® ACS, ISO, Reag. Ph. Eur. (Product No. 1.00063)
- Acetonitrile (gradient grade for liquid chromatography)—LiChrosolv® Reag. Ph. Eur. (Product No. 1.00030)
- Methanol (gradient grade for liquid chromatography)—LiChrosolv® Reag. Ph. Eur. (Product No. 1.06007)
- Potassium bromide for IR spectroscopy—Uvasol® (Product No. 1.04907)
- Purospher® STAR RP-18 endcapped (3 µm) 100 x 4.6 mm (Product No. 1.50469)
- Trifluoroacetic acid for spectroscopy—Uvasol® (Product No. 1.08262)
- Water for chromatography (LC-MS grade)—LiChrosolv® (Product No. 1.15333) or fresh water from the Milli-Q® system
- Aripiprazole United States Pharmacopeia (USP) Reference Standard (Product No. 1042634 USP)
- Aripiprazole Related Compound F United States Pharmacopeia (USP) Reference Standard (Product No. 1042689 USP)
To continue reading please sign in or create an account.
Don't Have An Account?