Protocol Guide: WST-1 Assay for Cell Proliferation and Viability
Protocols, FAQs and troubleshooting guide
Product No. CELLPRO-RO
Introduction
The measurement of cell proliferation and cell viability has become a key technique in the life sciences. The need for sensitive, reliable, fast and easy methods has led to the development of several standard assays. Proliferation assays have become available for analyzing the number of viable cells by the cleavage of tetrazolium salts added to the culture medium. The tetrazolium salts are cleaved to formazan by cellular enzymes (Figure 1). An expansion in the number of viable cells results in an increase in the overall activity of mitochondrial dehydrogenases in the sample, which in turn increases the amount of formazan dye formed. Quantification of the formazan dye produced by metabolically active cells can be done using a scanning multiwell spectrophometer.
The Cell Proliferation Reagent WST-1 is designed to be used for the non-radioactive, spectrophotometric quantification of cell proliferation, growth, viability, and chemosensitivity in cell populations using the 96-well-plate format. It can be used for,
- The measurement of cell proliferation in response to growth factors, cytokines and nutrients (Figure 2).
- The assessment of growth inhibitory antibodies and physiological mediators.
- Analysis of cytotoxic and cytostatic compounds, such as anti-cancer drugs and other pharmaceutical compounds.
![Cleavage of tetrazolium salt WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene sulfonate) to formazan. (EC = electron coupling reagent, RS = mitochondrial succinate-tetrazolium-reductase system). Cleavage of tetrazolium salt](/deepweb/assets/sigmaaldrich/marketing/global/images/technical-documents/protocols/cell-culture-and-analysis/cell-counting-and-health-analysis/formazen-dark-red/formazen-dark-red.png)
Figure 1.Cleavage of tetrazolium salt WST-1 (4-[3-(4-Iodophenyl)-2-(4-nitro-phenyl)-2H-5-tetrazolio]-1,3-benzene sulfonate) to formazan. (EC = electron coupling reagent, RS = mitochondrial succinate-tetrazolium-reductase system).
Kit Components (Catalog No. CELLPRO-RO)
The Cell Proliferation Reagent WST-1 is a clear, slightly red, ready-to-use solution, containing WST-1 and an electron coupling reagent, diluted in phosphate buffered saline, filtered through 0.2 µm pore size membrane.
Control (blank)
Add the same volume of culture medium and Cell Proliferation Reagent WST-1 as used in the experiment into one well (e.g., 100 µL cell culture medium plus 10 µl WST-1). Use this background control as a blank position for the ELISA reader. The background absorbance depends on the culture medium, the incubation time and exposure to light. Typical background absorbance after 2 h is between 0.1 – 0.2 absorbance units.
Assay Protocol to Measure Cell Proliferation
Determination of the activity of human interleukin-2 (hIL-2) activity on mouse T cell line CTLL-2 (93042610) (Figure 2).
Additional reagents required:
- Culture medium, e.g., RPMI 1640 (R0883) containing 10% heat inactivated FCS (fetal calf serum, 12106C) and 2 mM glutamine (G6392).
- Interleukin-2, human (hIL-2, SRP3085) sterile.
- If an antibiotic is to be used, additionally supplement media with penicillin/streptomycin or gentamicin.
Protocol:
- Seed CTLL-2 cells at a concentration of 4 × 103 cells/well in 100 μL culture medium containing various amounts of IL-2 (final concentration e.g., 0.005 – 25 ng/mL) into microplates (tissue culture grade, 96 wells, flat bottom).
- Incubate cells for 48 h at 37 °C and 5% CO2.
- Add 10 µL/well Cell Proliferation Reagent WST-1 and incubate for 4 h at 37 °C and 5% CO2.
- Shake thoroughly for 1 min on a shaker.
- Measure the absorbance of the samples against a background control as blank using a microplate (ELISA) reader. The wavelength for measuring the absorbance of the formazan product is between 420 – 480 nm (max. absorption at about 440 nm) according to the filters available for the ELISA reader. The reference wavelength should be more than 600 nm.
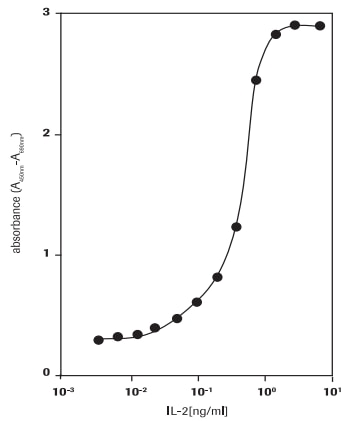
Figure 2.Measurement of proliferation of CTLL-2 cells in response to human IL-2 using WST-1 assay.
Assay Protocol to Measure Cytotoxicity
Determination of the cytotoxic effects of human tumor necrosis factor-α (hTNF-α, T6674) on WEHI-164 cells (mouse fibrosarcoma cell line, 87022501) (Figure 3).
Additional reagents required:
- Culture medium, e.g., RPMI 1640 (R0883) containing 10% heat inactivated FCS (fetal calf serum, 12106C), 2 mM glutamine (G6392) and 1μg/mL actinomycin C1 (actinomycin D, A9415).
- Tumor necrosis factor-α, human (hTNF-α, T6674) (10 μg/mL), sterile.
- If an antibiotic is to be used, additionally supplement media with penicillin/streptomycin or gentamicin
Protocol:
- Culture cells in microplates (tissue culture grade, 96 wells, flat bottom) in a final volume of 100 µL/well culture medium in a humidified atmosphere (37 °C and 5% CO2).
- Seed cells at a concentration of 5 × 104 cells/ well in 100 μL culture medium containing 1 μg/mL actinomycin C1 and various amounts of hTNF-α (final concentration e.g., 0.001–0.5 ng/mL) into microplates (tissue culture grade, 96 wells, flat bottom).
- Incubate cell cultures for 24 h at +37 °C and 5% CO2.
- Add 10 μL Cell Proliferation Reagent WST-1 and incubate for 4 h at 37 °C and 5% CO2.
- Shake thoroughly for 1 min on a shaker.
- Measure the absorbance of the samples against a background control as blank using a microplate (ELISA) reader. The wavelength to measure absorbance of the formazan product is between 420-480 nm according to the filters available for the ELISA reader (Figure 3). The reference wavelength should be more than 600 nm.
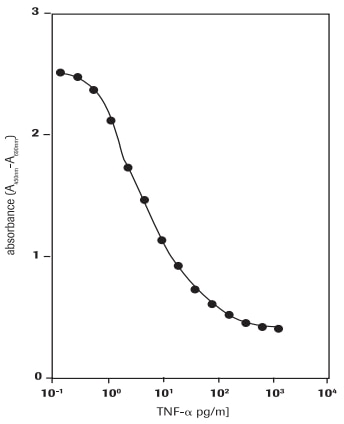
Figure 3.Determination of the cytotoxic activity of human TNF-α (hTNF-α) on WEHI-164 cells (mouse fibrosarcoma cell line) using WST-1 assay.
Frequently Asked Questions and Troubleshooting
1. Can I use the CELLPRO-RO kit with yeast or bacteria?
There is no data for using these kits for the measurement of bacterial or yeast proliferation; the kits have also not been developed for this purpose. The easiest way to measure bacterial or yeast proliferation would be to determine the OD.
2. Should I wait for cells to settle down before measurement?
Cells are not required to settle down to the bottom of the well as cells do not interfere with the measurement. Cells do not absorb, at the wavelength used for quantification of WST-1 cleavage. It is therefore possible to measure immediately after shaking without any further step, as described in the protocol in the package insert.
3. Can I simultaneously use the Cell Proliferation Reagent WST-1 and Cell Proliferation ELISA BrdU, colorimetric?
Figure 52a of the Roche ′Apoptosis, Cytotoxicity and Cell Proliferation Manual′, 4th edition shows the combined use of the Cell Proliferation Reagent WST-1 and the Cell Proliferation ELISA BrdU, colorimetric for simultaneous measurement of cell viability and cell proliferation. A protocol is given in the figure legend. To perform assays simultaneously one must order both kits, additional material is listed in the pack inserts.
4. Can I simultaneously use the Cell Proliferation Reagent WST-1, Cell Proliferation Kit I (MTT) and Cell Proliferation Kit II (XTT)?
The MTT/XTT/WST-1 assay utilize different tetrazolium salts to measure metabolic activity in active cells. Each kit requires a different wavelength to detect metabolic activity, MTT 550 nm, XTT 492 nm, and WST-1 420-480 nm. Discrimination of the signal from each salt would be difficult. Sensitivity and linear detection range of established kits run independently would be compromised with use of combined salts MTT, XTT, and WST-1. Therefore, simultaneous use of MTT, XTT, and WST-1 is not recommended.
5. How can I stop the WST-1 reaction?
Stopping the reaction of the Cell Proliferation Reagent WST-1 is possible by either freezing the sample or by adding 1% Triton X-100/ 0.1% SDS (final concentration) for 5 min at room temperature (+15 to +25 °C or +37 °C).
Troubleshooting
Potential interference of Phenol Red and/or FCS
Phenol Red will increase the absorbance by no more than 0.1 OD units; however, the negative controls make it possible to compensate for this. The addition of up to 10% FCS also causes no problems. Roche has not systematically tested the kit for a tolerance range for Phenol Red or FCS. Higher amounts of FCS should also cause no problems. In general, the negative control should always be treated in the same way as the samples.
Similar Assays
MTT assay and XTT assay can also be used for measuring cell viability and proliferation.
To continue reading please sign in or create an account.
Don't Have An Account?