MTT Assay Protocol for Cell Viability and Proliferation
Introduction
The MTT assay is used to measure cellular metabolic activity as an indicator of cell viability, proliferation and cytotoxicity. This colorimetric assay is based on the reduction of a yellow tetrazolium salt (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide or MTT) to purple formazan crystals by metabolically active cells (Figure 1).6,7,35 The viable cells contain NAD(P)H-dependent oxidoreductase enzymes which reduce the MTT to formazan.36 The insoluble formazan crystals are dissolved using a solubilization solution and the resulting colored solution is quantified by measuring absorbance at 500-600 nanometers using a multi-well spectrophotometer. The darker the solution, the greater the number of viable, metabolically active cells.
This non-radioactive, colorimetric assay system using MTT was first described by Mosmann, T et al.1 and improved in subsequent years by several other investigators.2-6 The Cell Proliferation Kit I (MTT) is an optimized MTT assay kit containing ready to use reagents, does not need washing steps or additional reagents. It is a quantitative assay that allows rapid and convenient handling of a high number of samples. The Cell Proliferation Kit I (MTT) can be used for multiple applications, such as,
- Quantification of cell growth and viability.1,3,5-7
- Measurement of cell proliferation in response to growth factors, cytokines and nutrients.1-3,6,8-12 (see fig. 3).
- Measurement of cytotoxicity. Examples are the quantification of tumor necrosis factor-a or -b effects 13,14 (see fig. 2) or macrophage induced cell death15,16 and the assessment of cytotoxic 17-34 or growth inhibiting agents such as inhibitory antibodies.
- To study cell activation.4
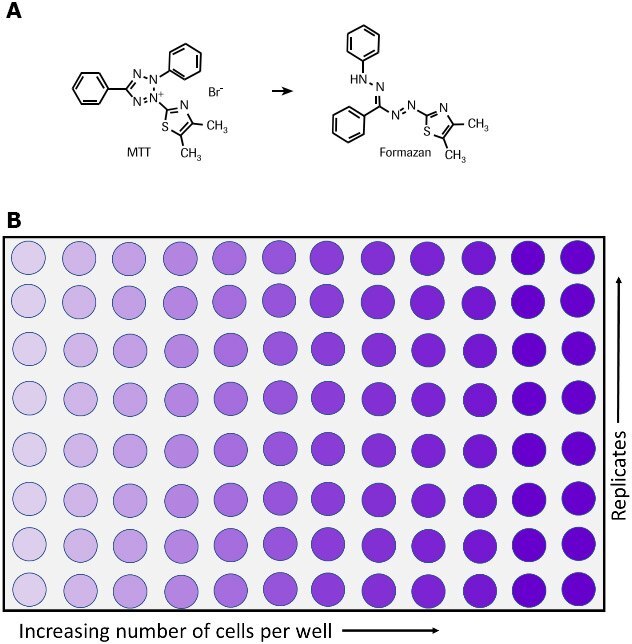
Figure 1.Metabolism of MTT to a formazan salt by viable cells as shown in a chemical reaction (A) and in a 96-well plate (B).
Kit Components (Catalog No. 11465007001)
MTT Reagent
- Ready to use, non-sterile
- 5 vials containing 5 ml MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) (1x), at 5 mg/ml in phosphate buffered saline (PBS, 806552)
Solubilization Solution
- 1x, ready-to-use
- 3 bottles with 90 ml.
Assay Protocol to Measure Cytotoxicity
Additional Reagents Required:
- Culture medium, e.g., RPMI 1640 (R0883) containing 10% heat inactivated FCS (fetal calf serum, 12106C), 2 mM glutamine (G6392) and 1μg/ml actinomycin C1 (actinomycin D, A9415).
- If an antibiotic is to be used, additionally supplement media with penicillin/streptomycin or gentamicin
- Tumor necrosis factor-α, human (hTNF-α) (10 μg/ml), sterile (T6674).
Protocol:
For the determination of the cytotoxic effect of human tumor necrosis factor-α (hTNF-α, T6674) on WEHI-164 cells (mouse fibrosarcoma, 87022501) (see fig. 2).
- Preincubate WEHI-164 cells at a concentration of 1 × 106 cells/ml in culture medium with 1 μg/ ml actinomycin C1 for 3 h at 37 °C and 5-6.5% CO2.
- Seed cells at a concentration of 5 × 104 cells/ well in 100 μl culture medium containing 1 μg/ml actinomycin C1 and various amounts of hTNF-α (final concentration e.g., 0.001–0.5 ng/ml) into microplates (tissue culture grade, 96 wells, flat bottom).
- Incubate cell cultures for 24 h at +37 °C and 5-6.5% CO2.
- After the incubation period, add 10 μl of the MTT labeling reagent (final concentration 0.5 mg/ml) to each well.
- Incubate the microplate for 4 h in a humidified atmosphere (e.g., +37 °C, 5-6.5% CO2).
- Add 100 μl of the Solubilization solution into each well.
- Allow the plate to stand overnight in the incubator in a humidified atmosphere (e.g., +37 °C, 5-6.5% CO2).
- Check for complete solubilization of the purple formazan crystals and measure the absorbance of the samples using a microplate (ELISA) reader. The wavelength to measure absorbance of the formazan product is between 550 and 600 nm according to the filters available for the ELISA reader, used. The reference wavelength should be more than 650 nm.
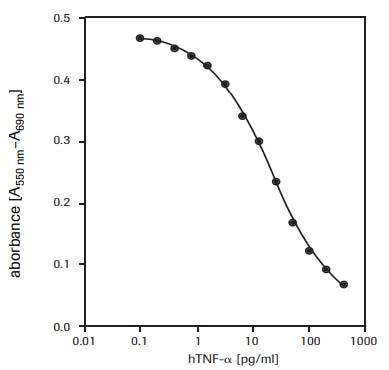
Figure 2.Determination of the cytotoxic activity of recombinant human TNF-α (hTNF-α) on WEHI-164 cells (mouse fibrosarcoma) using MTT assay.
Assay Protocol to Measure Cell Growth
Additional Reagents Required:
Culture medium, e.g., DMEM (D5671) containing 10% heat inactivated FCS (fetal calf serum, 12106C), 2 mM glutamine (G6392), 0.55 mM L-arginine (A8094), 0.24 mM L-asparagine-monohydrate (A4284), 50 μM 2-mercaptoethanol (M3148), HT-media supplement (H0137) (1×), containing 0.1 mM hypoxanthine and 16 μM thymidine. If an antibiotic is to be used, additionally supplement media with penicillin/streptomycin or gentamicin. Interleukin-6, human (hIL-6, SRP3096) (200,000 U/ml, 2 μg/ml) sterile.
Protocol:
For the determination of human interleukin-6 (hIL-6) activity on 7TD1 cells (mouse-mouse hybridoma, DSMZ, ACC 23) (see fig. 3).
- Seed 7TD1 cells at a concentration of 2 × 103 cells/well in 100 μl culture medium containing various amounts of IL-6 [final concentration e.g., 0.1-10 U/ml (0.001-0.1 ng/ml)] into microplates (tissue culture grade, 96 wells, flat bottom).
- Incubate cell cultures for 4 days at +37 °C and 5-6.5% CO2.
- After the incubation period, add 10 μl of the MTT labeling reagent (final concentration 0.5 mg/ml) to each well.
- Incubate the microplate for 4 h in a humidified atmosphere (e.g., +37 °C, 5-6.5% CO2).
- Add 100 μl of the Solubilization solution into each well.
- Allow the plate to stand overnight in the incubator in a humidified atmosphere (e.g., +37 °C, 5-6.5% CO2).
- Check for complete solubilization of the purple formazan crystals and measure the spectrophotometrical absorbance of the samples using a microplate (ELISA) reader. The wavelength to measure absorbance of the formazan product is between 550 and 600 nm according to the filters available for the ELISA reader, used. The reference wavelength should be more than 650 nm.
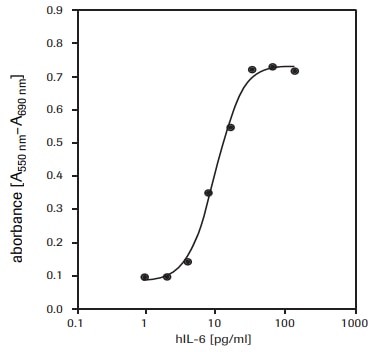
Figure 3.Proliferation of 7TD1 cells (mouse-mouse hybridoma) in response to recombinant human interleukin-6 (hIL-6) using MTT assay.
Similar Assays
XTT assay and WST-1 assay can also be used for measuring cell viability and proliferation.
References
To continue reading please sign in or create an account.
Don't Have An Account?