Creating Transgenic Mice using CRISPR-Cas9 Genome Editing
Although several genome editing tools have been developed in recent years, including zinc finger-based strategies and TALENs (transcription activator-like effector nucleases), none have been as efficient as the CRISPR/Cas9 system, which consists of an RNA-guided DNA endonuclease (Cas9) and corresponding guide RNAs (CRISPRs). Using this system, researchers were able to achieve one-step generation of mutant mice with both alleles of multiple genes being knocked out.1 Mice that bore conditional alleles and reporter genes were also created,2 and the protocol required as fast as two or three weeks. Notably, the procedure does not require the sometimes-arduous process of creating modified mouse ES cells.3 With the development of the Cas9 knock-in and knock-out mouse, it is expected that more and more laboratories will be choosing the CRISPR/Cas9 system for generating transgenic mouse models.
Protocol for Creating Transgenic Mice using CRISPR-Cas9
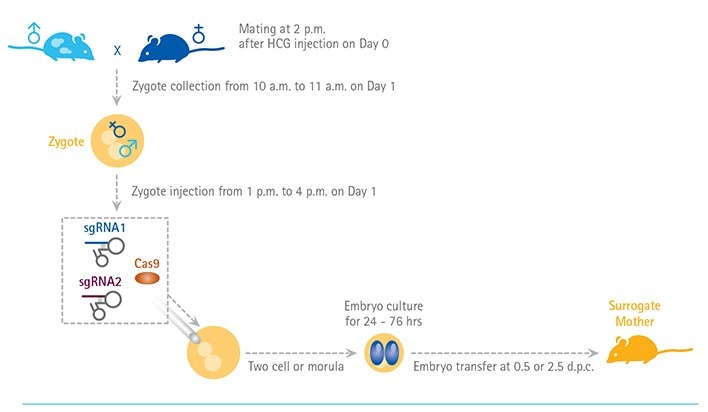
Figure 1. Schematic of using CRISPR/Cas9 genome editing on mouse embryos to create transgenic mice. By coinjecting Cas9 mRNA and guide RNAs multiple gene targets can be knockout at once in mouse embryos. (Adapted from Yang H, Wang H, and Jaenisch R. Nat Protoc. 2014 Aug;9(8):1956-68.)
We provide tools and custom services for genome editing, including ZFN and CRISPR/cas9. We also offer an extensive portfolio of mouse embryo-validated media and reagents for the storage, transfer and expansion of mouse embryos used to create transgeneic mouse models under the EmbryoMAX™ name.
Browse all Mouse Embryo Validated Reagents
Tips for a Successful Mouse Model Project
1. Know the purpose of the experiment and do your research.
Generating the right mouse requires fully understanding the hypothesis being tested. For example, an investigator might wish to test the hypothesis that mutating a transporter protein in the liver might mitigate the hepatotoxic effects of a particular drug. Knowing this hypothesis will help the team select the appropriate mouse strain and design appropriate targeting vectors and biological controls. Thoroughly read the literature and all mouse genetics informatics databases to determine if an appropriate mouse model already exists, and if so, whether it can be obtained from the creators.
2. Determine the spatial and temporal environment in which gene expression changes should occur.
In light of the hypothesis being tested, determine whether the gene modification should be controlled by a tissue-specific, lineage-specific or inducible promoter. Some transgenes or gene knockouts may be lethal to the embryo; these are cases in which inducible promoters are required.
3. Choose an appropriate mouse strain.
Each strain of inbred mice has distinguishing characteristics and may be uniquely suited to particular areas of research. For example, BALB/c mice are suitable for immunology research, but, due to inherent neurodevelopmental defects, may not be suited for generating models of nervous system disorders. NOD mice are suitable for models of autoimmunity and metabolic syndrome, but, due to their short lifespan, have limited utility for studies of aging.
4. Use well-characterized promoters and design restriction sites into the construct so that you have the option of removing vector sequences that inhibit transgene expression.
If expressing an exogenous gene, ideally, the targeting vector should include a promoter that has previously been used to generate transgenic mice. Also, try to determine if vector sequences might inhibit expression. Always test the construct in cultures of the cell type of interest to confirm expression.
5. Include a strong polyadenylation sequence (such as SV40 polyadenylation sequence).
Include an intron sequence in the construct so that the transcript will be spliced. Because the splicing machinery may mediate transcription-translation coupling, gene expression may be enhanced by inclusion of an intron.
6. Optimize rate of homologous recombination.
Increasing the length of the homologous region in your targeting construct may increase the rate of recombination. The chromatin structure of your target may affect recombination frequency, as well as genetic background.
7. If transfecting ES cells, use a high-efficiency transfection reagent.
Try numerous Transfection Reagents or methods to determine which generates transfected ES cell clones with the greatest efficiency and lowest toxicity.
8. Establish a screening assay, such as a PCR assay or Southern blot.
Ideally, your screening assay should employ a probe or primers bearing sequences not present in the unmodified cell.
9. Develop an assay for gene expression.
Potential assays include Western Blot, ELISA, Luminex® assay, immunohistochemistry, flow cytometric assay or RT-PCR.
10. Develop a reliable genotyping strategy.
Using tail biopsies or ear punch tissue, develop a rapid protocol for isolating genomic DNA and assessing genotype using PCR. Some DNA polymerases, such as KOD Xtreme™ DNA polymerase, are particularly suited to genotyping, because they can accurately and efficiently amplify DNA from crude samples, such as tail digests. For such polymerases, it may not be necessary to purify the tail digest before adding 1 μL or less of the digest to the PCR reaction. Tail biopsies frequently yield large amounts of DNA; if PCR results in no visible bands, it may be that an overabundance of template sequence compared to primer concentration is inhibiting amplification.
11. Follow best practices for breeding transgenic mice to maintain stocks.
The number and characteristics of breeding pairs required to maintain your supply of modified mice depend on many factors. Some of these factors include strain-specific behavior, effects of the particular mutation or transgene, colony temperature and humidity, light source and light cycle, vibrations, noise, odors, handling, nutrition, barometric pressure, and overall health.
References
To continue reading please sign in or create an account.
Don't Have An Account?