Certified Reference Materials for 19F Quantitative NMR Ensuring Traceability to “The International System of Units” (SI)
Romana Rigger, Alexander Rück, Christine Hellriegel, Robert Sauermoser, Fabienne Morf, Kathrin Breitruck, Markus Obkircher
In recent years quantitative NMR (qNMR) spectroscopy has become one of the most important tools for content determination of organic substances and quantitative evaluation of impurities. The implementation of qNMR for new application fields, e.g. metabolomics, environmental analysis, and physiological pathway studies, brings along more complex molecules and systems, thus making the use of 1H-qNMR challenging. A smart workaround is possible through the use of other NMR active nuclei, namely 31P and 19F.
At our manufacturing site in Buchs (Switzerland), we have been using qNMR since 2009 to produce certified reference materials (CRM) traceable to the SI unit, under ISO/IEC 17025 and ISO Guide 34 (since 2017: ISO 17034) accreditation (an example of a traceability chain is shown in Figure 1). The TraceCERT® product range of organic CRMs suitable for HPLC or GC is certified using this technique and comprises over 200 products including pesticides, vitamins, amino acids, plasticizers, PAHs, antibiotics, FAMEs and many other product groups. In addition to this product range, we also provide a toolkit of qNMR standards traceable to primary material from NIST (National Institute of Standards and Technology, USA) or NMIJ (National Metrology Institute of Japan), see SigmaAldrich.com/qnmr. The expansion of this qNMR standard product line with new, interesting CRMs is ongoing and up-to- date 16 different 1H qNMR CRMs with known purity values and small expanded measurement uncertainties have been developed. They cover the whole spectral and solubility range, enabling access to the qNMR certification of hundreds of organic products.
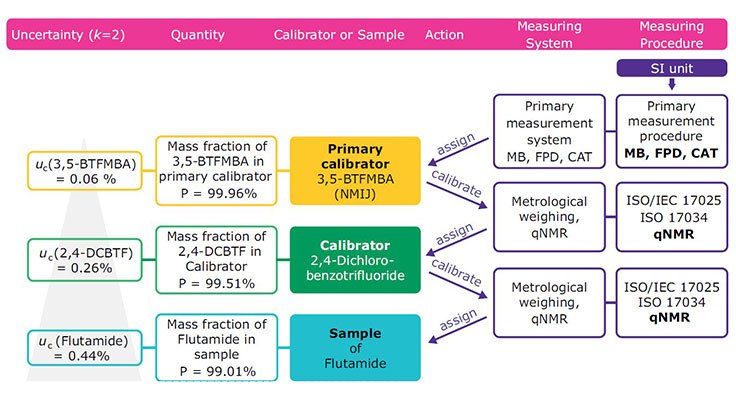
Figure 1.Traceability chain of Flutamide. Certification was done by comparison with 2,4-DCBTF (secondary calibrator) and 3,5-BTFMBA (primary calibrator) and finally to the SI unit. MB = mass balance, FPD = freezing point depression, CAT = coulometric acidimetric titration.
In certain cases, 1H qNMR reaches its limits, especially regarding the certification of complex and larger molecules. However, new fields of application often also bring along the presence of heteroatoms, namely 31P and 19F. Thus we introduced 4 CRMs for 31P qNMR with traceability to the SI.
In the following section, the development of CRM for the use in 19F qNMR is described. This article is an excerpt from our AOAC paper published in 2017. Please refer to this reference for further information.1
3,5-Bis(trifluoromethyl)benzoic acid (3,5-BTFMBA, NMIJ CRM 4601-a) is a primary CRM for use in 1H and 19F qNMR certified by NMIJ. The NMR shift range of 19F is very large but the window for linear excitation, which is necessary for 19F qNMR, is quite small and depends on field strength and NMR parameter. Techniques to counter this dilemma were published earlier including the use of new NMR experiments. Therefore, we set out to develop qNMR CRMs with peaks in different shift regions which can further be chosen corresponding to the analytes’ shift and employed in standard 19F qNMR experiments. Two of the most common structure elements are CF3 groups and fluorine atoms bound directly to substituted aromatic compounds. Shifts of 19F in CF3 groups arise around -55 to -90 ppm, while shifts of fluorine atoms bound to aromatics can be found between approximately -110 and -180 ppm. Further structure elements show signals between -70 and -140 ppm (CF2) or between -120 and -240 ppm (fluorine atoms in saturated and unsaturated aliphatic compounds).
Recently three different 19F qNMR CRMs were developed by us. They were selected based on various parameters including solubility, stability, homogeneity, purity and shift range. As a prerequisite to show traceability to the SI and the certification concept, we selected molecules that carry both 1H and 19F nuclei. 2,4-Dichlorobenzotrifluoride (2,4-DCBTF, cat. no. 53396) is liquid and the CF3 group shows a singlet at -61.2 ppm in the 19F spectrum, depending on the solvent (DMSO-d6 ). The three aromatic protons show analyzable signals between 7.5 and 8.5 ppm in the 1H spectrum (DMSO-d6). 2-Chloro-4-fluorotoluene (2Cl4FT, cat. no. 80730) is also liquid and the fluorine atom bound to the aromatic ring shows a multiplet at -115.3 ppm
(DMSO-d6) in the 19F spectrum. In the 1H spectrum, again three aromatic protons show peaks between 7.0 and 8.0 ppm and an additional peak can be found for the methyl group at around 2.3 ppm (DMSO-d6). 4,4’-Difluorobenzophenone
(4,4’-DFBP, cat.no. 07563) is solid and the two symmetrical fluorine atoms show a multiplet at around -106.5 ppm
(DMSO-d6) in the 19F spectrum. Eight aromatic protons give signals between 7.0 and 8.0 ppm (DMSO-d6). All three compounds are soluble in common organic NMR solvents. Molecular weights are 215 g/mol (2,4-DCBTF), 144.57 g/mol (2Cl4FT) and 218.2 g/mol (4,4’-DFBP). Purity values, expanded measurement uncertainties, NMR solvent specific shifts and relaxation times (T1) can be found in Table 1.
Technical aspects of 19F qNMR
A characteristic of 19F NMR is given by 13C and 12C satellites that are present in the NMR spectrum. The interaction of 19F with 12C and 13C leads to an isotopic effect and thereby to unsymmetrical satellites on the one hand and to multiple satellites around the main peak in non-decoupled spectra on the other hand. Additionally, peak shapes are different depending on the structure element. In general, CF3 peaks show singlet signal pattern and aromatic bound 19F atoms multiplet signal pattern.
As with 31P qNMR, inverse gated decoupling was used during 19F qNMR data acquisition. Using this method instead of an e.g., power-gated decoupler minimizes NOE (Nuclear Overhauser Effect) build-up. With this experiment, decoupling is applied only during data acquisition and thus allows the spin system to reach equilibrium between decoupling steps. By applying inverse gated decoupling, only one satellite appears that is on only one side of the main peak (Figure 2). When performing pretests, a set of decoupled and coupled spectra was recorded to distinguish between satellites and impurities. Integration of decoupled spectra (Figure 2) was done either including both satellites, only the 12C satellite, or if possible no signals between 7.5 and 8.5 ppm in the 1H spectrum satellite. No matter which possibility was chosen, integration was performed in the same way for the internal standard and the sample compound with regard to the line width. Similar to 13C, the 19F nucleus has a wide chemical shift range. To perform quantitative measurements, broadband excitation over the full spectral width is required. Due to insufficient available radiofrequency power for pulsed excitation, signal intensities and thus signal integration can be error-prone. The effect leads to relatively narrow ranges of frequencies (15 - 30 kHz, 600 MHz NMR, 90° pulse) where an accurate quantification can be guaranteed. This requires sound pretesting, followed by accurate adjusting of spectral width and transmitter frequency offsets. Furthermore it is important to set the acquisition time as short as possible to avoid NOE build up, but long enough to avoid loss of spectral quality by truncation of the Free Induction Decay (FID). That requires an additional analysis of the FID prior to quantitative measurements. All 19F NMR experiments were performed on a Bruker Avance III 600 MHz NMR instrument equipped with a Prodigy TCI probe head.
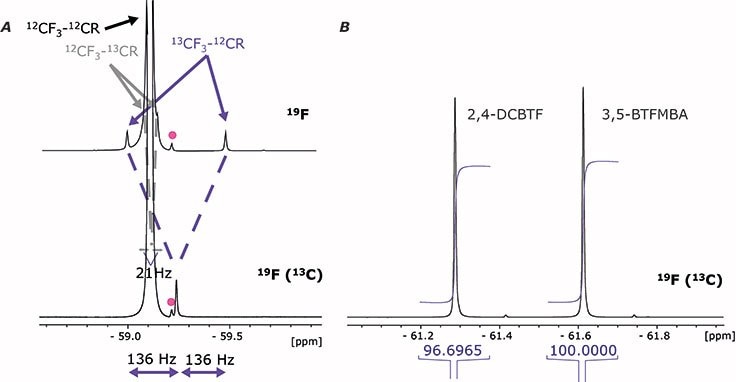
Figure 2. A: 19F NMR Signal of Flutamide in coupled (19F) and decoupled (19F(13C)) spectra. The pink point is an impurity. Satellites are assymetrically arranged around the main peak in coupled spectra. B: Example for the integration of 2,4-DCBTF (analyte) and 3,5-BTFMBA (internal standard). Both signals were integrated without the outer satellite.
Even though a standard probe (instead of a dedicated 19F probe) was used, a good spectral quality could be ensured. Background distortions by probe head and sample tube materials, pulse breakthrough and ringing artifacts influence the spectral quality, especially the baseline (rolling baseline), which is typical for 19F, 11B and 29Si and increases when measuring over large spectral width. This can be counteracted by either applying additional processing steps (FID repair by cutting the first data points before data transformation) or by increasing the pre-scan delay. For 19F qNMR experiments during the development of our CRMs, an increased pre-scan delay was used and no FID cutting was done. T1 times were determined by inversion recovery experiments. Typical T1 times for 19F qNMR CRMs are between 1.2 and 4.8 s depending on the concentration, of the mixture and solvent. Multiplying T1 times by a factor of 7-10 gives D1 times between 20 and 35 s.
CRM for 19F qNMR - traceability to the SI through primary CRM
Similar to the study published for 31P, a traceability scheme for 19F qNMR CRMs was elaborated to guarantee the traceability to the SI unit and show the comparability of 1H and 19F qNMR experiments and thus the independency of the result of the measured nucleus (Figure 3, C). As primary reference material, 3,5-BTFMBA of the National Metrology Institute of Japan was selected. This reference is highly pure (99.96 %), has a very small expanded measurement uncertainty (0.06 %, k=2) and the two symmetrical CF3 groups show a sharp 19F signal at -61.3 ppm (in DMSO-d6). The three aromatic protons give signals around 8.2 - 8.6 ppm (DMSO-d6), depending on the solvent. 3,5-BTFMBA is soluble in all common organic solvents and is specified by NMIJ for 1H and 19F qNMR.

Figure 3.Traceability chains for 1H (A), 31P (B) and 19F (C) qNMR CRMs. Pink arrows symbolize 1H qNMR measurements, blue arrows 31P measurements and green arrows 19F qNMR measurements. Light grey boxes indicate primary reference material, dark grey boxes 1HTraceCERT® qNMR CRM, dark blue and dark green boxes 31P and 19F TraceCERT® qNMR CRM and light blue and light green boxes testing substances (chromatography TraceCERT® CRM).
The purity value of 2,4-DCBTF was certified by 19F and 1H qNMR using 3,5-BTFMBA. In a second way, certification was done with 1H qNMR using 1,2,4,5-Tetrachloro-3-nitrobenzene (TCNB, cat. no. 40384) with traceability to the primary CRM BA (NIST SRM® 350b). The three purity values and their expanded measurement uncertainties are in perfect accordance (SD = 0.015, Figure 4). Values for uc(CRM) (k=2) are also comparable between the different experiments (0.25 – 0.29 %).
Due to different signal shapes and spectral regions of peaks, 2Cl4FT and 4,4’-DFBP were certified by another route. Traceability to the SI for 2Cl4FT was achieved by determining a mass fraction via 1H qNMR using 3,5-BTFMBA. In a second way, Benzyl benzoate (BBO) was used as internal standard. A third value is assigned by 19F qNMR using 4,4’-DFBP as internal standard. The purity values from the three different measurements are overlapping within their expanded measurement uncertainties and again show good accordance (SD = 0.053, Figure 4). The uncertainty values uc(CRM) (k=2) are similar to that of 2,4-DCBTF (0.24 – 0.41 %).
The purity value of 4,4’-DFBP was certified via 1H qNMR using 3,5-BTFMBA, and in a second way Maleic acid (MA, cat.no. 92816), as internal standard. 19F qNMR certification was performed using 2Cl4FT, showing again the independency of the result of the observed nucleus. All three values are comparable and the SD of the three results is small (SD = 0.055, Figure 4). The values of uc(CRM) are slightly higher compared with the other two 19F qNMR CRMs (0.30 to 0.37 %). The increased uncertainties (e.g., 0.41 %, 2Cl4F2 and 0.37 % 4,4’-DFBP) do not result from the measurement procedure but are caused by a higher uncertainty contribution by the internal standard (4,4’- DFBP, MA) and homogeneity of the material. In all other 19F certifications the overall repeatability of the measurement represents the most significant uncertainty contribution.
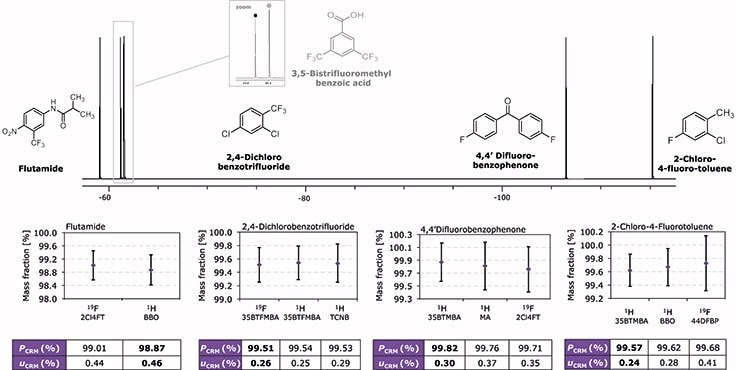
Figure 4. 19F spectrum showing shifts of different secondary CRMs and the primary CRM 3,5-BTFMBA. Depending on the structure element (CF3 or aromatic bound F) peaks are shifted to different regions. Results for the purity determination of secondary CRMs via 19F and 1H qNMR and using different internal standards are shown. Different values for purity of an analyte (PCRM) are within their expanded measurement uncertainties uCRM. The certified values for 2,4-DCBTF, 4,4’-DFBP and 2Cl4FT are shown in bold, and CRM 4601-a was selected as the primary CRM for the three. Due to chemical shifts in the NMR spectrum, direct comparison on the basis of 19F was only possible in the case of 2,4-DCBTF. In the cases of 4,4’-DFBP and 2Cl4FT, 1H qNMR had to be used, but also referencing to CRM 4601-a. Flutamide could be measured in both ways.
A last experiment was done to assign a purity value to the TraceCERT® Flutamide CRM. It was possible to show, that via 19F qNMR and using 2,4-DCBTF as internal standard, comparable results were achieved as by the common route via 1H qNMR using an established CRM (BBO). Again, overall repeatability of the measurement represents the most significant uncertainty contribution, which is in the same order for certification via 1H and 19F. The purity values are overlapping within their expanded measurement uncertainties (Figure 4), which is again a clear indicator that 19F qNMR can be used routinely as a stand-alone method to assign the purity of fluoroorganic substances.
Conclusion
In summary, qNMR using 1H, 31P, or 19F TraceCERT® CRMs is a very valuable method. We outlined sensitive aspects that are important for an accurate qNMR certification and need particular awareness by the operator. The presented set of 1H, 31P, and 19F qNMR CRMs is produced fulfilling the requirements for a reference material producer under ISO 17034 accreditation, covering additional data such as homogeneity of the material and short-term and long-term stability.
References
To continue reading please sign in or create an account.
Don't Have An Account?