Assessing Enriched Murine CD4+ T Cells Differentiated Towards Effector T Helper Cell Lineages with the Scepter™ 2.0 Handheld Automated Cell Counter
Introduction
Cellular differentiation, the process by which progenitor cells differentiate into specialized cell types, is fundamental to developmental biology. In the immune system, immune progenitor cells must differentiate into diverse innate and adaptive phenotypes for the body to respond to infections. In the adaptive immune system, CD4+ T cells can give rise to a variety of effector T, or “T helper” cell subsets, depending on the nature of the immune response, and subsequently release a distinct set of cytokines. During T cell differentiation, T helper (Th) cell subsets plays a critical role in the immune response repertoire, defending the body against foreign pathogens.
The major subsets of differentiated CD4+ T cells include Th1, Th2, Th17, Treg, and Tfh cells. Each subtype expresses a signature set of cytokines and/or transcription factors that directs the immune response and regulates differentiation (Figure 1). For example, Th1 CD4+ T cells are important for protecting against intracellular bacteria, fungi, and viruses, as well as being involved in some autoimmune responses. These cells mediate immune responses to intracellular pathogens by producing interferon gamma (IFN-γ), and via its secretion, activate macrophages, natural killer (NK) cells, and CD8+ T cells. In contrast, Th2 CD4+ T cells are often associated with responses during which high levels of pathogen-specific immunoglobulin are generated to neutralize large, extracellular pathogens. Th17 cells play an important role in the induction and propagation of autoimmunity. The signature cytokine of this population is IL-17, whose expression has been associated with diseases such as multiple sclerosis, rheumatoid arthritis, psoriasis, and inflammatory bowel disease as well as in allergic responses.
As CD4+ T cells differentiate towards a specific effector T cell lineage, the cells change dramatically in size — a unique physical hallmark of this differentiation process. We hypothesized that using the Scepter™ cell counter to rapidly assess size distributions of cellular populations would provide a quick, simple method for tracking T cell differentiation.
The Scepter™ cell counter combines the ease of automation with the accuracy of impedance-based counting using the Coulter principle in an affordable, handheld format. The instrumentation has been collapsed into a device the size of a pipette, and uses a combination of analog and digital hardware for sensing, signal processing, data storage, and graphical display in the form of a histogram. The histogram output provides a quick snapshot of cell size and density.
In this study, we demonstrate a method for driving murine CD4+ T cell differentiation towards Th1, Th2, or Th17 cells in vitro and measuring cell size changes before and after this process, using the Scepter™ cell counter. We have employed Scepter™ technology for cell volume/size determination to investigate the relationship between cell differentiation and cell size changes. We confirmed T cell differentiation status by using a flow cytometry assay to measure intracellular cytokine production for Th1, Th2, and Th17 cell types. Although the Scepter™ cell counter was intended here primarily as a cell counting device, we also demonstrate how this instrument can function as a reliable tool for other, diverse biological applications.

Figure 1. Effector CD4+ T cell lineage commitment. CD4+ T cells can give rise to many subtypes, depending on the required immune response. A complex process that includes activation of T cell receptors, along with a local cytokine environment, polarizes CD4+ T cells to a defined lineage of mature cytokine-producing helper T cells and Treg cells.
Materials and Methods
Enriched CD4+ T cell isolation
Mouse spleens were harvested from 8-10 week old C57BL/6 mice (Charles River Laboratories) and prepared according to the standard guidelines for obtaining single-cell suspensions (splenocyte harvesting). Homogenized spleens were passed through a nylon mesh to ensure single-cell suspensions, followed by treatment with red blood cell lysing buffer (Sigma®, Catalog No. R7757) to deplete red blood cells from the culture. Cells were gently re-suspended in Hank’s Balanced Salt Solution (HBSS; Sigma®, Catalog No. H9394), counted to determine splenocyte recovery, and then applied to a CD4 isolation column (R&D Systems, Catalog No. MCD4C-1000) to enrich for CD4+ T cells.
CD4+ T Cell Differentiation Towards Specific Effector T Cell Lineages
Mouse CD4+ splenocytes were differentiated to one of three distinct lineages through a process of activation, expansion, and re-stimulation. The cells were activated by culturing on anti-CD3 coated plates with the single addition of a combination of growth factors and antibodies in precise amounts to begin the differentiation process (Catalog No. FCIM025161 for Th1 cells; Catalog No. FCIM025162 for Th2 cells; Catalog No. FCIM025163 for Th17 cells). Cells were expanded and then re-stimulated at days 4 and 6, respectively. After day 6, the differentiated Th cell cultures were secreting their signature cytokines, as verified by flow cytometry.
Scepter™ Cell Counting
The Scepter™ cell counter was used to count samples following the detailed on-screen instructions for each step of the counting process. Briefly, the user attaches a 40 μm sensor tip, depresses the plunger, submerges the sensor into the sample, then releases the plunger, drawing 50 μL of cell suspension into the sensor. The Scepter™ device detects each cell passing through the sensor’s aperture, calculates cell concentration, and displays a size-based histogram as a function of cell diameter or volume on its screen. Scepter™ 2.1 software was then used to upload test files from the device and perform subsequent data analysis to determine cell sizes for mouse CD4+ T cell (undifferentiated) versus Th1, Th2, or Th17 effector T cell cultures (differentiated).
Evaluation of Th1, Th2, and Th17 Cell Lineages by Flow Cytometry
All flow cytometry assays were performed using MilliporeSigma’s FlowCellect® Mouse Th intracellular cytokine kits (Catalog No. FCIM025123 for Th1 cells; Catalog No. FCIM025124 for Th2 cells; Catalog No. FCIM025125 for Th17 cells). All reagents and detailed instructions are provided in the user guides for each kit. Please refer to each kit’s specific instruction for more detailed information. All sample acquisition and data analysis was performed using the Guava® easyCyte 8HT system.
Comparison of Scepter™ Cell Analysis with an Automated Image-based Cell Viability System
Using the convenient and cost-effective Scepter™ cell counter, cell sizes of undifferentiated CD4+ T cells and Th1 CD4+ T cells were analyzed in triplicate assays (three independent experiments, three samples per experiment) and standard deviation values were determined. In parallel, both sets of samples were analyzed using a more expensive automated cell viability analyzer.
Results
Mouse CD4+ T cells were treated with established lineage-specific factors for six days to drive differentiation towards effector T cell-specific subsets: Th1, Th2, or Th17 cells. Using the MilliporeSigma mouse differentiation tool kits as described previously, we were able to successfully achieve cell differentiation in an in vitro system, and these results are captured using the Scepter™ device, based on cell size discrimination. Cross-validation studies were performed by flow cytometry using MilliporeSigma's mouse intracellular cytokine kits to verify results.
Scepter™ Analysis of CD4+ T Cell Differentiation Toward the Th1 Lineage
Before induction, undifferentiated CD4+ T cells were measured using the Scepter™ cell counter, and demonstrated a mean cell diameter of 6.4 μm (Figure 2, blue histogram). After six days in culture under the influence of Th1 phenotype-inducing reagents such as lineage-specific cytokines and growth factors, the resulting Th1 cells were measured using the Scepter™ cell counter. The mean cell diameter of this differentiated cell population was 10.4 μm (Figure 2, yellow histogram). Figure 3 shows brightfield microscopy analysis of the same cells, confirming that their average diameter had indeed increased.
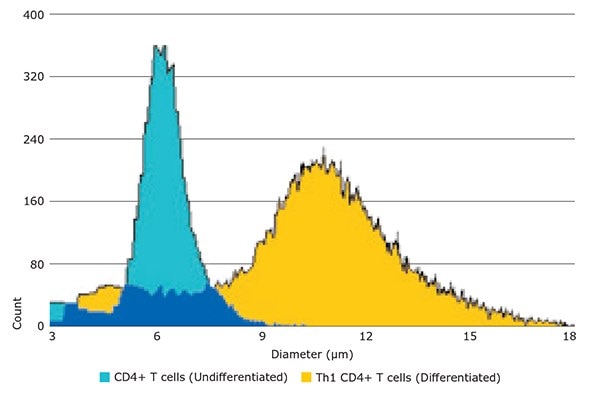
Figure 2.Accurate discrimination between mouse CD4+ T cells
Validation of Th1 Cell Differentiation Using Flow Cytometry
In order to validate that the cell types measured were truly differentiated cells, a flow cytometry assay was performed to measure intracellular cytokine production. As shown in Figure 4, 91.2% of the day 6-stimulated cell population expressed IFN-γ, a characteristic Th1 cell marker, confirming that the increase in cell size observed in figures 2 and 3 was indeed a phenotype of differentiated Th1 cells.
The Scepter™ cell counter precisely measured cell diameter changes across multiple samples (Table 1). Moreover, the data showed that the Scepter™ cell counter could achieve similar or more precise measurements when compared to an alternative benchtop instrument.
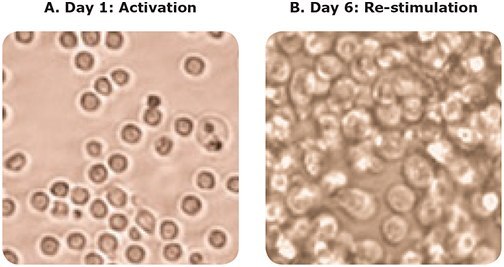
Figure 3. Microscopy images before and after T cell differentiation: Confirmation that cell size changes are detectable by the Scepter™ cell counter. CD4+ T cells prior to treatment under differentiation conditions exhibited cell diameters around 5-6 μm, as shown in (A). In (B), six days after exposure to specific cytokines and growth factors to induce differentiation towards the Th1 cell lineage, cells appear much larger when visualized at the same magnification.
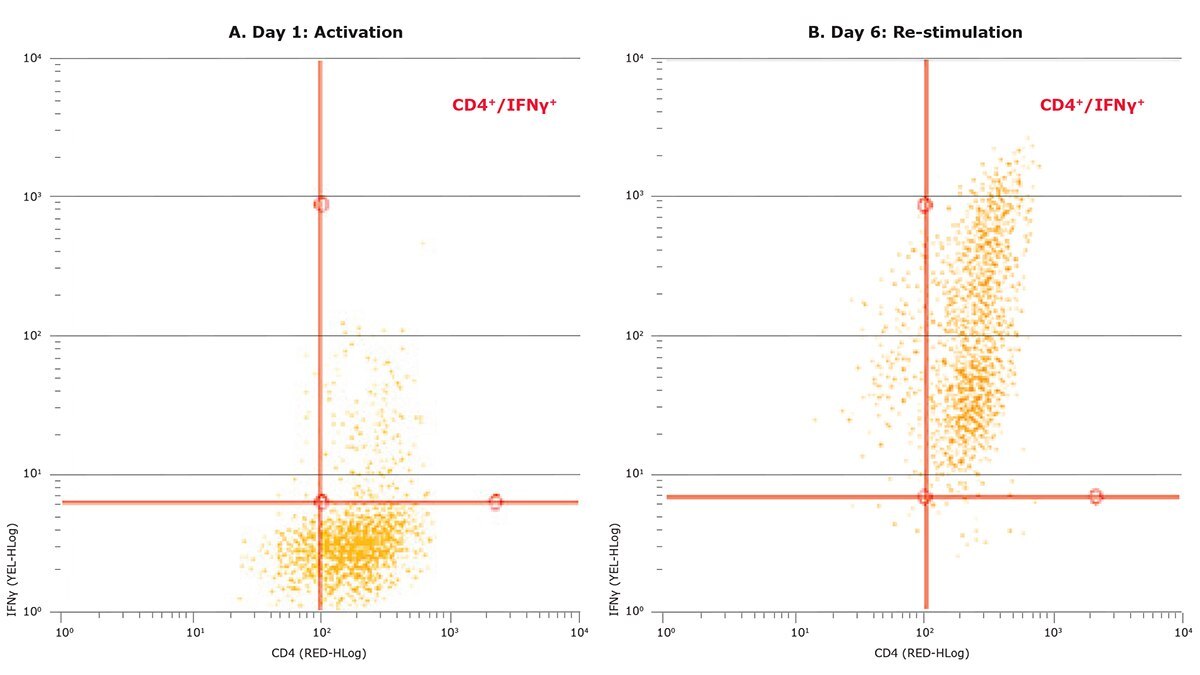
Figure 4. Flow cytometry validates differentiation towards Th1 cells, confirming results obtained using the Scepter™ cell counter. Using the FlowCellect® Mouse Th1 Intracellular Cytokine Kit (Catalog No. FCIM025123), percentages of differentiated versus undifferentiated CD4+ T cells were determined. Undifferentiated cells did not show significant expression of the signature cytokine IFN-γ (A); after six days in culture under lineage-specific differentiation treatment toward Th1 cells, cells showed positive expression of IFN-γ (B).
CD4+ T Cell Differentiation Toward the Th2 and Th17 Lineages
CD4+ T cells were induced to differentiate towards both Th2 and Th17 cell lineages using MilliporeSigma's Mouse Th Differentiation Tool kits for Th2 and Th17 cells (Catalog No. FCIM025162 and FCIM025163, respectively). Before induction, undifferentiated CD4+ T cells were measured using the Scepter™ cell counter and found to have a mean cell diameter of 6.3 μm (Figure 5, blue histogram). After six days in culture under the influence of lineage-specific reagents, cells were differentiated towards the Th2 and Th17 cell types. The resulting cell populations were then measured using the Scepter™ cell counter, which reported mean cell diameters of 10.1 μm and 9.8 μm for Th2 and Th17 cells, respectively (Figure 5, yellow histograms).
In order to validate that the cell types measured were truly differentiated cells and specific for each cell type described, we used flow cytometry to measure intracellular cytokine production for cells exposed to each induction treatment. As shown in Figure 6, increased expression of IL-4 for Th2 cells and increased expression of IL-17 for Th17 cells confirmed that the observed increases in cell size correlated with phenotypes of differentiated cells.
As with Th1 cell differentiation, the Scepter™ cell counter precisely measured cell diameter changes across multiple samples of progenitor T cells and those differentiated toward Th2 and Th17 lineages (Table 2). Once again, data demonstrated that the Scepter™ cell counter could achieve more precise measurements when compared with an alternative benchtop instrument.
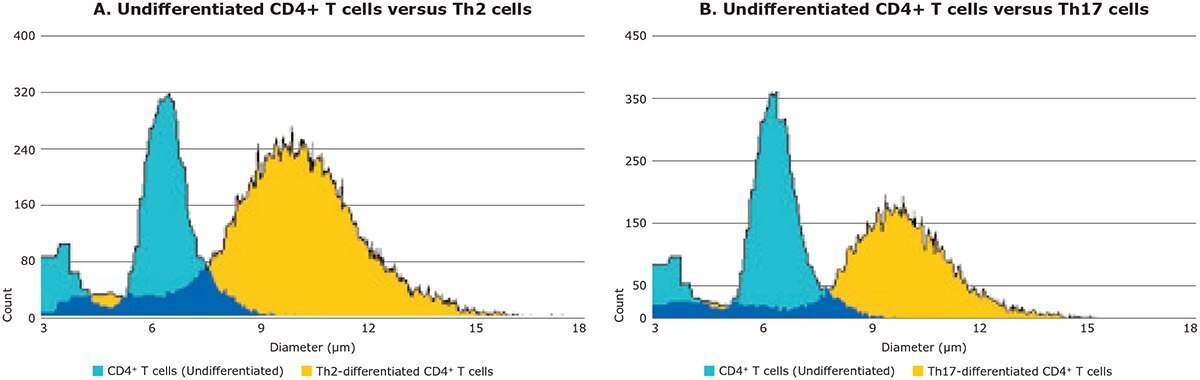
Figure 5. CD4+ T cell differentiation toward Th2 and Th17 cell lineages can be clearly identified based on cell size using the Scepter™ cell counter. Using a 40 μm sensor, the Scepter™ cell counter enables the discrimination of cell types based on size, with high resolution. The size distributions of Th2 and Th17 cell populations were compared to the size distribution of the CD4+ T cell progenitor cell type. Both Th2 and Th17 cells gradually increased in size from ~6 to about 10 μm after six days of differentiation.
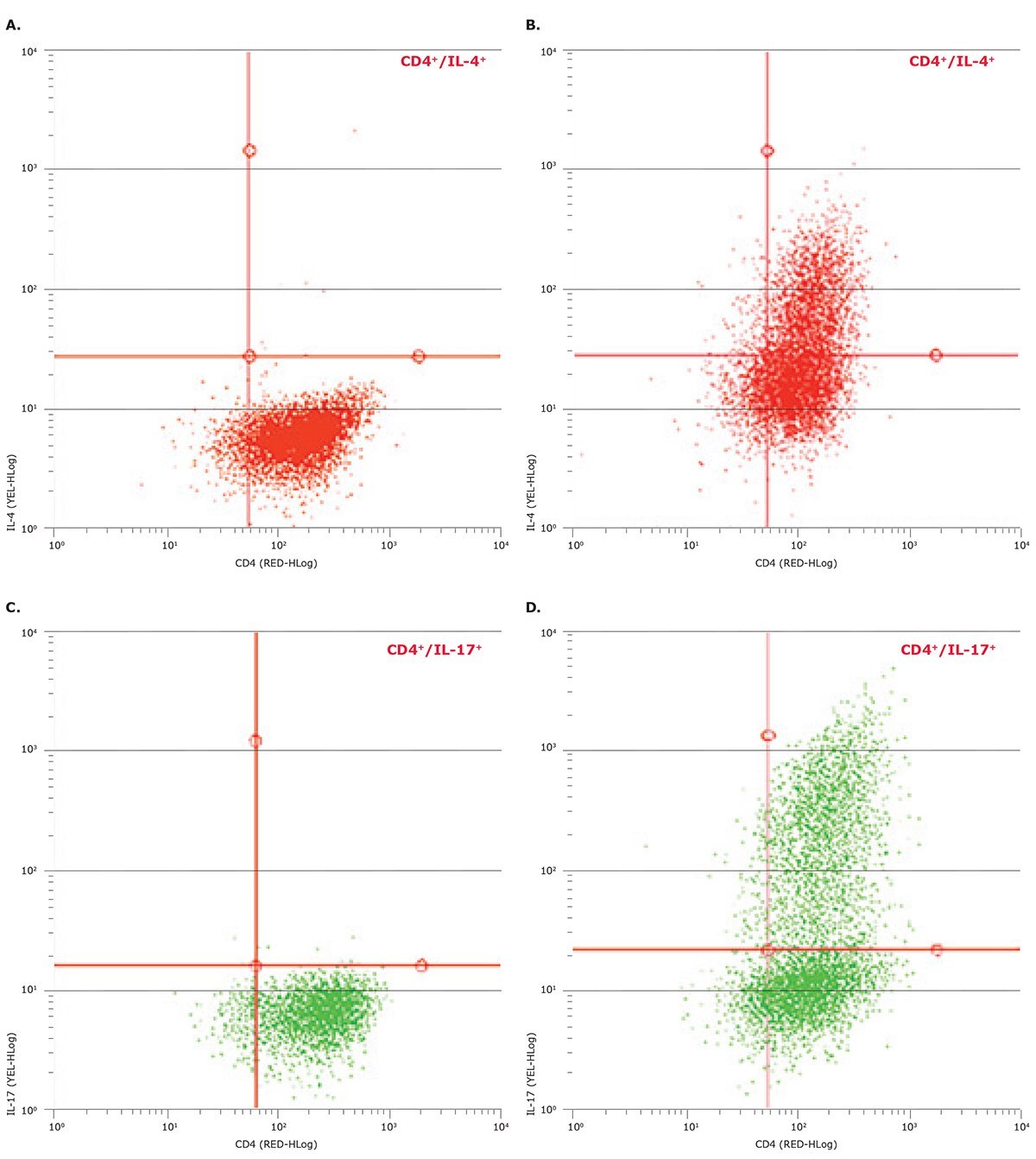
Figure 6. Flow cytometry confirmation of the differentiation of enriched CD4+ T cells to Th2 and Th17 cells. Cell types that demonstrated larger cell diameters as detected by the Scepter™ cell counter had been cultured for six days as shown in (B) and (D), whereas their progenitor cell types are shown in (A) and (C). Differentiation-treated cells in (B) and (D) expressed the signature cytokines IL-4 and IL-17 for Th2 and Th17 subtypes, respectively. Table 1 and Table 2 were summarized in a bar graph (Figure 7) showing the clear size difference between undifferentiated and differentiated CD4+ T cell populations, underscoring the utility of the Scepter™ cell counter to provide a rapid, simple, accurate method for tracking immune response.
Th2 cell phenotype |
|---|
Th17 cell phenotype |
|---|
Conclusions
T cell differentiation and its role in the immune response are integral for both innate and adaptive immunity. The importance of effector T cells and their ability to combat various types of pathogens, as well as maintaining immune homeostasis, is a hallmark of the complexity of mammalian immune systems. The process of differentiation into T helper (Th) cell subsets specialized for diverse pathogens and functions is key to a varied and effective immune response. Dysregulated Th cell function often leads to inefficient clearance of pathogens, which can cause diseases of inflammation and may also impact autoimmunity.
Differentiation of CD4+ T cells into fully functional effector T cells is characterized by the production of signature cytokines and a concomitant increase in cell size. As demonstrated in this study, Th1, Th2, and Th17 cells expanded from approximately 6 to 10 μm when compared to the unstimulated CD4+ T cell phenotype. This expansion upon differentiation was simply and accurately measured using the Scepter™ cell counter.
The Scepter™ cell counter provides a rapid, easy, and inexpensive method for assessing naive CD4+ T cell differentiation toward specific effector T cell lineages. This handheld, automated cell counter delivers precise, reliable cell size measurements, which can provide the researcher with a quick snapshot of differentiation status for T cells and other immune cell types. The methods described here provide a new, rapid method for distinguishing cell phenotype, while simultaneously distinguishing accurate cell quantitation.
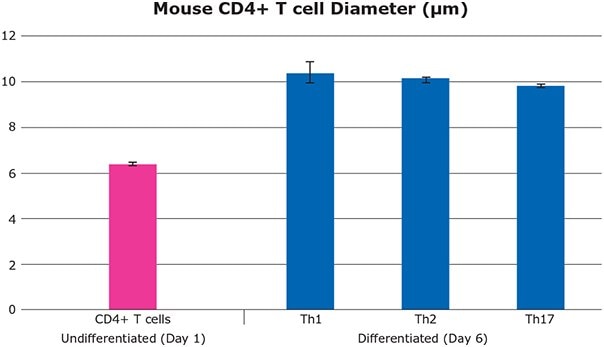
Figure 7. Cell diameter change during CD4+ T cell differentiation (toward Th1 / Th2 / Th17 cell lineages) as measured by the Scepter™ cell counter. Featured Pro
References
To continue reading please sign in or create an account.
Don't Have An Account?