Precise and Accurate Bead Counting Using the Scepter™ Handheld Automated Cell Counter
Introduction
Micron-sized beads are used in a variety of biological applications, ranging from daily validation of flow cytometer performance, to purification of fusion protein constructs from cell lysates. Depending on the nature of the assay, the beads may either possess magnetic properties or be labeled internally with a fluorescent dye. In bead-based multiplexed immunoassays, these particles are coated with unique recognition molecules, such as epitope-specific antibodies, permitting capture and precise quantification of desired analyte(s). Accurate determination of bead counts at the onset of each assay allows for standardization of bead concentration across multiple samples, and minimizes errors and variation in downstream results.
A number of methodologies are currently available for particle counting. While manual counting using a hemocytometer is accessible and inexpensive, it is laborious and error-prone due to user subjectivity. Automated counting devices are commercially-available in two formats: vision-based platforms and flow-based systems. Most vision-based counters use the standard Trypan blue exclusion assay to assess viability, and employ a digital camera with image analysis software to determine particle size and concentration.1 Flowbased devices measure particles in a stream using impedance-based detection. By precisely controlling the flow, volumetric measurements can be obtained, thereby permitting estimation of sample bead concentration.2 For most researchers, a critical barrier to using an automated vision-based or flow-based system is the price associated with large benchtop instruments.3
With the Scepter™ cell counter, we have combined the ease of automated instrumentation with the accuracy of impedance-based counting using the Coulter principle in an affordable, handheld tool. The instrumentation has been collapsed into a device the size of a pipette, and uses a combination of analog and digital hardware for sensing, signal processing, data storage, and graphical display. The 40 μM and 60 μM aperture sensors are engineered with a microfabricated sensing zone that enables discrimination by bead size and bead volume at submicron and subpicoliter resolution, respectively Table 1 outlines the specifications for each sensor type.
While the Scepter™ device was initially optimized for cell counting, herein we report that the device is also well suited for the precise counting of diverse types of beads commonly employed in other biological applications.
Materials and Methods
Bead counting using the Scepter™ cell counter
Sample preparation
Bead suspensions (Table 2 for list of beads tested) were serially diluted in phosphate-buffered saline (PBS, Product No. BSS-1006-A). The concentration range tested (50,000 to 1,500,000 beads/mL), corresponds to the upper and lower limits of detection for the 40 μm sensor. PBS is at an optimal salt concentration for appropriate conductivity that is required for accurate counting performance. For each test, we used the recommended sample volume of 100 μL in a 1.5 mL microcentrifuge tube. Other tubes may not be able to accommodate the width of the sensor, or may not provide sufficient sample depth for the instrument to function properly. Since beads can settle quickly, we kept the bead suspension well mixed prior to testing ensuring reproducible counts.
Bead counting
Operation of the Scepter™ cell counter is similar to using a standard laboratory pipette. The Scepter™ cell counter is turned on by depressing and holding the toggle on the back of the instrument. Once on, the instrument will prompt the user to attach a sensor. The Scepter™ unit displays detailed on-screen instructions for each step of the counting process. Briefly, depress the plunger and submerge the tip into the solution. Next, release the plunger to draw 50 μL of bead suspension into the sensor. The Scepter™ cell counter detects each particle passing through the sensor’s aperture, then calculates concentration and displays a histogram of bead diameter or volume on its screen.
Bead types tested
Scepter™ Software
The upper and lower limits of the histogram, called gates, are either set automatically based on the histogram profile, or can be set to the same gates used in the previous count. After the count is complete and the histogram is displayed on the instrument, the gates can be moved manually to fine-tune the analysis. Up to 72 histograms can be stored on the instrument. All test data files can be uploaded to a computer and further analyzed using Scepter™ Software Pro.
Bead counting by other methods
For some tests, counts were also performed using the Z2 Coulter CounterR (Beckman Coulter) and a visionbased automated cell counter. Counts were performed according to the manufacturer’s instructions using the same starting suspension and serially diluted samples.
Results
Precision counting with Scepter™ cell counter
A variety of bead types ranging in size from 4 - 10 μM were counted using the Scepter™ counter fitted with 40 μM sensors. For each bead type, the original sample was diluted to an approximate concentration of 1.5 x 106 beads/mL and counted using a Coulter CounterR fitted with a 50 μM aperture to determine the theoretical starting concentration. Next, a serial dilution series (2-fold) was prepared and assayed to determine theoretical concentrations at each dilution step. All seven bead types tested yielded interpretable histograms that could be gated and used to calculate sample bead size and concentration. Examples of these histograms are shown in Figure 1.

Figure 1.Histogram overlays showing serial dilution of two bead types. Scepter™ Software Pro displays imported size distribution histograms as either a single sample histogram or as overlaid histograms for multiple samples. Shown are overlaid histograms for serially diluted 5.6 μM MILLIPLEXR map microspheres and 4.5 μM Invitrogen Dynabeads®.
To validate the reproducibility of measured concentration values, we plotted the mean derived concentration values (n=4) versus their theoretical values across the dilution range. The bead counting precision using the Scepter™ counter was determined by calculating coefficients of variation at each data point, for each bead type individually (Figure 2 and Table 3). As shown, the overlap of data points and small error bars suggest that, regardless of bead type, concentration values were accurate, precise, and reliable up to a concentration of 1.5 x 106 beads / mL. The highest variability, both within and between bead types, was found at 1.5 x 106 beads/mL which coincides with the upper detection limit of detection for the 40 μm sensor. Overall, the high degree of linearity (as shown by R2 values) indicates that counting with the Scepter™ cell counter is a reliable method for the bead types tested, across a wide linear operating range.
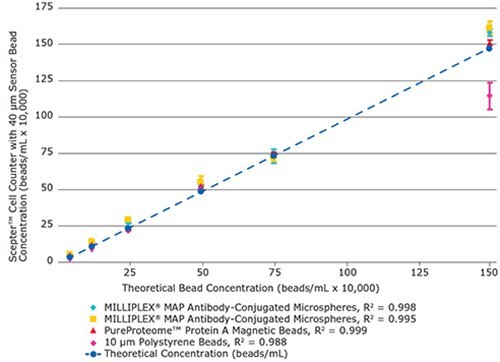
Figure 2.The Scepter™ cell counter performs with high linearity (R2 ≥ 0.99) across multiple, diverse bead types, over a wide operating range. Shown here are bead concentration data for four representative samples out of seven bead types tested.
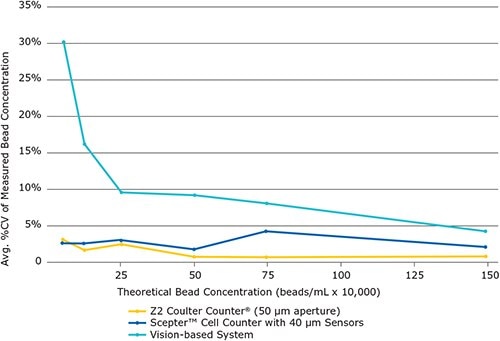
Figure 3.The Scepter™ cell counter performs bead counting with smaller coefficients of variation (%CV) than vision-based, automated counting. Shown are the average %CVs of three bead types (MILLIPLEXR map Antibody-Conjugated Microspheres, MILLIPLEXR map Antibody Conjugated Magnetic Microspheres and PureProteome™ Protein A Magnetic Beads) with respect to bead concentration and counting method.
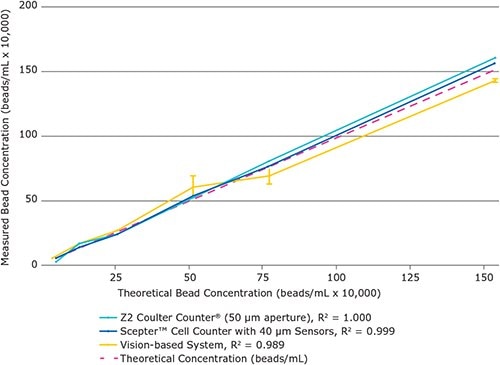
Figure 4.The Scepter™ cell counter counts PureProteome™ Protein A magnetic beads with greater linearity and smaller standard deviation than visionbased automated counting. Beads were counted using the methods shown. Data points represent average of four replicates. Error bars represent standard deviation.
Comparative platform analysis
Comparing the coefficients of variation (%CV) values for the Scepter™ cell counter, the Z2 Coulter CounterR, and the vision-based cell counter acquired during measurement of the serial dilution samples revealed that the Scepter™ cell counter was only slightly less precise than the gold standard Z2 Coulter CounterR instrument but significantly more precise than the vision–based automated counting system (Figure 3). Furthermore, the Scepter™ cell counter data displayed smaller standard deviations than the vision-based counting system data for all bead types and concentrations tested (Figure 4).
Size measurement
For the Scepter™ cell counter, the ability to accurately determine particle size is an inherent property of the sensor’s aperture. For example, the 40 μM sensor is capable of sizing particles in the 4-16 μM range. To determine the accuracy in reporting bead size, we compared our results to the known bead diameter values (obtained from the certificates of analysis) for two bead types. Calibrated latex microparticles (5 μM and 10 μM) were measured and results are shown in Figure 5. Accurate sizing was observed using both the Scepter™ cell counter and the Coulter CounterR instrument.
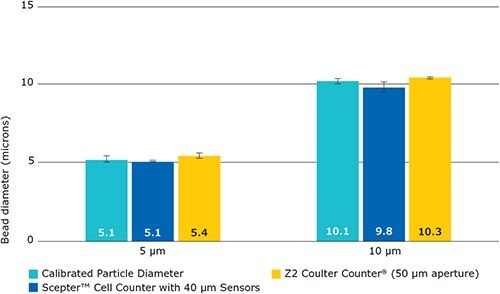
Figure 5.The Scepter™ cell counter and the Z2 Coulter CounterR accurately measure bead diameter. The average diameters of serially diluted samples of calibrated latex microparticles (5 μM and 10 μM) were measured with the Scepter™ cell counter and Coulter Counter®. Measured diameters were averaged across all samples within the counting range (50000-1500000 beads/mL).
Discussion
Comparing the performance of the Scepter™ cell counter to results from other counting methods, we conclude that this handheld, automated cell counter delivers precise, fast, and reliable bead count and size measurements over a wide operating concentration range. The superior functionality of the Scepter™ cell counter is due to the sensor's precision-engineered technology and sophisticated, Coulter principlebased counting instrumentation. High performance quality combined with it's convenient and intuitive form, suggest that the Scepter™ handheld counter will quickly be integrated into the standard workflow of researchers who need to enhance the efficiency of rudimentary bead counting and improve the reproducibility of bead-based assays, such as immunoprecipitation and multiplexed detection.
References
To continue reading please sign in or create an account.
Don't Have An Account?