Article Discussing Fatty Tissue Fixation Using Microwave Technology in Hematology and Histology Research.
Mary Faith Abbuhl, MS HTL(ASCP)QIHC Andrea Williams, HT(ASCP) University Hospitals of Cleveland Cleveland, OH Mary.Abbuhl@uhhs.com
This article has been reprinted with permission from HistoLogic, vol 38 no. 1, 2005.
Abstract
The microwave oven has been in use in the histology laboratory for many years. In recent years, laboratory-grade microwave instruments have become more widely adopted as standard equipment in many laboratories.1 These microwave units may be used to accelerate a number of laboratory procedures, including fixation, processing, decalcification, special staining, and immunohistochemical (IHC) staining.
Our histology laboratory has been using microwave technology for special stain procedures and antigen retrieval techniques in immunostaining procedures. Most recently, we investigated the use of the microwave to aid in fixation of fatty tissue specimens. These tissue specimens often do not process well during routine tissue processing.2 Tissue thickness and fat content are two main factors causing the problem of underprocessed tissue.
Introduction
Diagnostically, the most critical fatty tissues are breast and lymph node specimens. Any delay in processing these specimens may affect patient care. We are continually faced with inadequate processing of fatty tissues, most often due to both the thickness of the sections when grossed in and their fat content. The use of alcoholic formalin in preprocessing and processing, often recommended as a solution to this problem, is not an option for some immunohistochemical procedures, especially those typically used for breast samples. We have found that formalin fixation done in a microwave oven offers a suitable alternative when preparing fatty samples.
Materials and Methods
For this study we collected a limited number of samples (n=20) of breast tissue from mastectomies and breast reductions. Parallel sections of these tissues were cut in at the grossing table at three thicknesses—approximately 3 mm, 5 mm, and 6 mm—and placed into plastic tissue cassettes. The cassettes were then placed and held in 10% formalin (Val Tech Diagnostics, Brackenridge, PA).
One group of cassettes was processed routinely using a Tissue-Tek® VIP 3000 processor (Sakura Finetek, Inc.,Torrance, CA), while parallel blocks were fixed in a MicroMED T/T™ microwave oven (Hacker Instruments,Winnsboro, SC) followed by routine processing in the VIP 3000 processor. The cassettes to be microwaved were placed into the MicroMED T/T cassette holder in a large (400 ml) glass container that was filled with for a 5 minute ramp-up of temperature of the formalin to 60°C, and this temperature was maintained with microwave stimulation for 55 minutes at 60°C. The microwaved tissues were then transferred to the routine processor.
Tissue blocks were routinely embedded in paraffin and sectioned.3 Each sample was stained with H&E (Richard Allan Scientific, Kalamazoo, MI) and Masson's trichrome (Poly Scientific R&D Corp., Bayshore, NY). In addition, IHC was performed for estrogen receptors (ER), progesterone receptors (PR), and HER-2/neu (Hercep Test, DakoCytomation, Carpinteria, CA).
Results
A preliminary study of 20 patient samples was conducted. These specimens were all extremely fatty tissues. Tissues of 3 mm thickness or less consistently processed well after microwave fixation, requiring no additional processing, while routinely fixed 3 mm sections did not consistently process well in the routine processor. Many required reprocessing. The tissues of greatest thickness, approximately 6 mm, did not process well, even after microwave fixation. The tissues of intermediate thickness, approximately 5 mm, were inconsistent in processing, irrespective of the method employed for fixation.
We chose 3 samples to evaluate staining consistency when comparing tissues fixed in the microwave to those fixed routinely. H&E and trichrome stains, as well as three immunostains, were compared for each (Table 1). With respect to nuclear detail, the microwave fixed tissue showed superior staining with H&E (Figs. 1 and 2). In many cases it was necessary to cut the routinely fixed tissue thicker in order to obtain a better section, due to the tissue’s fat content. Overall, microwave fixed tissue could be sectioned thinner, thereby yielding a higher quality sample to view microscopically. The trichrome stain displayed similar results, however, the nuclear staining appeared slightly paler but crisper in the microwave fixed tissue (Figs. 3 and 4).
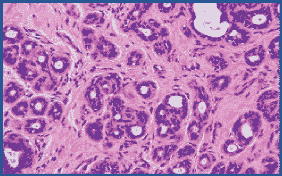
Figure 1. Routinely fixed and processed breast tissue stained with H&E. 200X
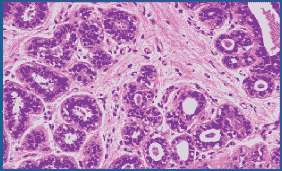
Figure 2:Microwave fixed breast tissue stained with H&E. Nuclear detail is improved. 200X

Figure 3. Routinely fixed and processed breast tissue stained with Masson’s trichrome. 200X
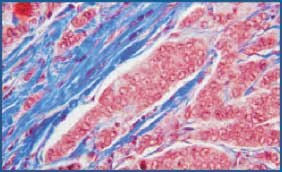
Figure 4.Microwave fixed breast tissue stained with Masson’s trichrome. Note the crisp nuclear detail. 200X
Immunostaining analysis of ER and PR receptors and HER-2/neu showed comparable staining (Figs. 5-8 ). Nonspecific background staining seen in the routinely fixed tissue was completely absent in the microwave fixed tissue. Only one sample showed very rare PR staining in the routinely fixed tissue but not in the microwave fixed tissue.This discrepancy may be attributable to sampling but warrants further study. None of the tissues studied was found to be positive for HER-2/neu, preventing us from evaluating the quality of immunostaining with this marker. Although the tissues were negative with this antibody, nonspecific background staining commonly seen in the routinely fixed tissues was virtually absent in the microwave fixed tissues.
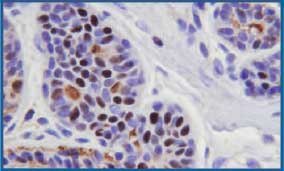
Figure 5. Routinely fixed and processed breast tissue stained with IHC for estrogen receptor protein. Some background staining is noted in intercellular areas and ductal lumens. 400X

Figure 6. Microwave fixed breast tissue stained with IHC for estrogen receptor protein. Tissue shows minimal background staining. 400X

Figure 7:Routinely fixed and processed breast tissue stained with IHC for progesterone receptor protein. 400X

Figure 8. Microwave fixed breast tissue stained with IHC for progesterone receptor protein. 400X
Conclusion
Historically, we have found that routine fixation and processing of even the thinnest of fatty tissue sections frequently requires reprocessing in our laboratory. Even though the number of specimens observed in this study is very small, we believe that formalin fixation of fatty tissue specimens processed using microwave technology, especially with tissues sectioned at a reasonable thickness, helps to prevent the need for reprocessing of these tissues.
Microwave fixation of fatty tissues does not appear to create any undesirable artifacts with H&E, trichrome, or immunohistochemical staining. On the contrary, it appears to help decrease nonspecific IHC background staining seen in some routinely prepared sections and improve the nuclear detail of the cells overall.
References
To continue reading please sign in or create an account.
Don't Have An Account?