Renal Transplant Rejection
Ranjitha Veerappan, MD, Lou Ellen Miller, HT, HT (ASCP), Piyush Joshi, MD, George A. Youngberg, MD
This article has been reprinted with permission from HistoLogic, vol 37 no. 2, 2004.
Introduction
Determining if the cause of renal allograft dysfunction in the early post transplant period is due to rejection or other reasons is a daunting task for the clinician. Clinical signs and symptoms alone are not helpful in clearly distinguishing the numerous causes of renal dysfunction. Various noninvasive tests including imaging studies have also been unsuccessful in distinguishing rejection from other causes.1 Renal allograft biopsy has, therefore, emerged as a key element in aiding in the accurate diagnosis of transplant rejection.
An allograft biopsy in the context of acute rejection not only helps to confirm the diagnosis, but it also reveals if the rejection is a result of humoral (antibody –mediated) or cellular immunologic mechanisms. This distinction determines the therapeutic course and the successful reversal of acute rejection. Cellular rejection is the more common type and is typically manifested by a T-lymphocyte attack directed against renal tubules (and occasionally against blood vessels). Microscopically, the early stages are characterized by edema and focal infiltration of the interstitium and peritubular capillaries by lymphocytes. In later stages, plasma cells, monocytes, and macrophages appear along with a more diffuse lymphocytic infiltrate. Invasion of the tubular epithelium by lymphocytes is a characteristic finding of tubulointerstitial rejection.2 To standardize diagnoses and avoid false positives, a formal system for interpreting renal allograft biopsies was developed.3 In this system, the diagnosis of acute cellular tubulointerstitial rejection is based on the finding of tubulitis. The criterion for diagnosing tubulitis is the identification of intraepithelial lymphocytes in the tubules.
The interpretation of tubulitis has traditionally been done using periodic acid-Schiff hematoxylin (PASH) staining, which clearly identifies the tubular basement membranes and, therefore, aids in the accurate assessment of the location of the lymphocytes (inside versus adjacent to the tubule). However, a significant drawback related to the PASH stain is that intraepithelial lymphocytes cannot always be reliably distinguished from tubular epithelial cell nuclei. Additional stains such as leukocyte common antigen (LCA) or a T-cell stain (UCHL-1) that specifically identifies lymphocytes have been used to address this issue. However, these stains again raise the issue of the location of the lymphocytes (inside versus adjacent to the tubule), since the basement membrane cannot be visualized by these methods. The ideal stain, therefore, would be the one that combines the ability of the PASH stain to visualize the basement membrane and also identify the lymphocytes with a specific marker such as LCA or UCHL-1. This method would greatly facilitate and improve the accuracy of diagnosing acute cellular tubulointerstitial rejection. Such a stain has recently been developed4 and can be produced using the Ventana Benchmark™ automated immunohistochemistry staining system and the NexES Special stains instrument.
Case Report
A 32-year-old man with a live unrelated donor renal allograft was noted to have rising creatinine. His immunosuppressive regimen consisted of rapamycin and steroids, with no mycophenolate mofetil or calcineurin inhibitors. The elevated creatinine did not respond to pulse methylprednisolone, so a percutaneous renal biopsy was obtained.
Methods
Routine light microscopic renal biopsy stains, including PASH and Hematoxylin & Eosin (H&E), were made using standard methods. A UCHL-1/PASH stain was prepared according to the method of Resch et. Al,4 using a Ventana (Ventana,Tucson, AZ) combined automated immunohistochemical (Benchmark™) and histochemical (NexES) stainer. Briefly, the renal core biopsy was received in Carson’s fixative, processed overnight, and paraffin embedded. The sections were cut at 3 microns and stained on the Benchmark™ using the iView DAB paraffin detection kit (Ventana,Tucson, AZ) with the T-cell (CD45RO) antibody. After the antibody step, the slides were removed from the Benchmark™, rinsed in DI water/ Dawn dishwashing liquid, and placed on the NexES for routine PASH stain. The slides were then dehydrated through graded alcohols, cleared in xylene, and mounted with a permanent mounting medium.
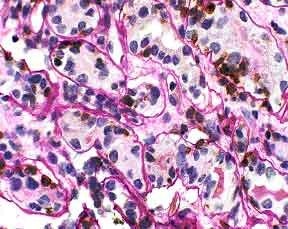
Figure 1. Tubulitis in the renal biopsy. Lymphocyte cytoplasm stains brown. Tubular borders are clearly delineated. UCHL-1/PASH 400x
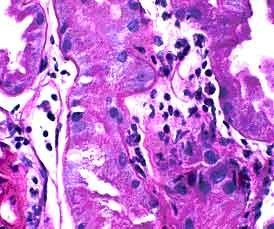
Figure 2. Distinguishing lymphocytes from tubular cells can be difficult on a standard PASH stain. PASH 400x
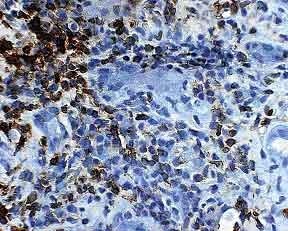
Figure 3. It is difficult to identify borders of tubules on a standard lymphocyte stain; determining the location of the lymphocytes (outside versus inside the tubular basement membranes) can be problematic. LCA 400x
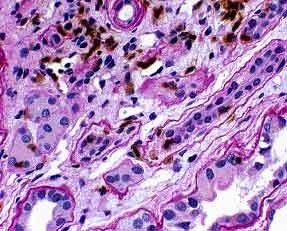
Figure 4. Lymphocytes are clearly identified, and their precise location in relation to tubular basement membranes is easy to determine on the PASH/T-cell stain. UCHL-1/PASH 400x
Results
The biopsy demonstrated moderate interstitial inflammation involving greater than 25% of the cortical area. The inflammatory infiltrate consisted predominantly of lymphocytes, with some admixture of plasma cells, eosinophils, and neutrophils. Several tubular profiles showed greater than four mononuclear cells per tubular cross-section. This was most clearly demonstrated on the PASH/T-cell stain (Figure 1). No tubular cross sections contained more than ten mononuclear cells. The PASH/T-cell stain confirmed that the majority of lymphocytes present were T-cells. A diagnosis of acute cellular rejection, tubulointerstitial type was made (Banff 1997 acute rejection, type IA).
Discussion
The key element in making a diagnosis of acute cellular rejection, tubulointerstitial type, is the identification of tubulitis. This can be challenging because lymphocytes in foci of presumptive tubulitis can be difficult to distinguish from tubular nuclei on standard stains like H&E or PASH (Figure 2). Immunohistochemical stains for LCA or T-cell markers can reliably identify the lymphocytes, but it may be difficult for these stains to distinguish whether the lymphocytes are truly inside the tubular basement membrane or merely adjacent to the tubule (Figure 3). Combining a basement membrane stain (PAS) with a T cell stain (UCHL-1) elegantly solves the problem of both specifically identifying T-lymphocytes and precisely determining their location (Figure 4). Thus, the presence of tubulitis can quickly and accurately be confirmed or excluded. This combination of UCHL-1/PASH greatly improves the accuracy of diagnosing of acute cellular tubulointerstitial rejection and helps determine the therapeutic course and prognosis.
References
To continue reading please sign in or create an account.
Don't Have An Account?