IVD Lateral Flow Assay Development
Making IVD Lateral Flow Test Strips for Analytes
Lateral flow test strips based on the principles of immunochromatography exist for a wide array of target analytes. The first tests were made for the detection of human chorionic gonadotropin (hCG). Today, commercially available tests monitor ovulation, detect infectious disease organisms, analyze drugs of abuse, and measure other analytes important to human physiology. Products have also been introduced for veterinary testing, agricultural applications, environmental testing, and product quality evaluation. While the first tests presented qualitative results based on the presence or absence of a signal line, test design has progressed toward semi-quantitative and quantitative assays and the integration of hand-held readers.
Most lateral flow test strips are modeled after existing immunoassay formats. Thus, test strips for hCG are typically sandwich assays; and drugs of abuse are analyzed by competitive or inhibition assays. In serum assays, antibodies are detected as indicators of various disease states. Many variations are possible, but they all have in common the formation of a complex between a detector particle that is free in the sample stream and a capture reagent that is bound to the membrane at the test line (Figure 1).
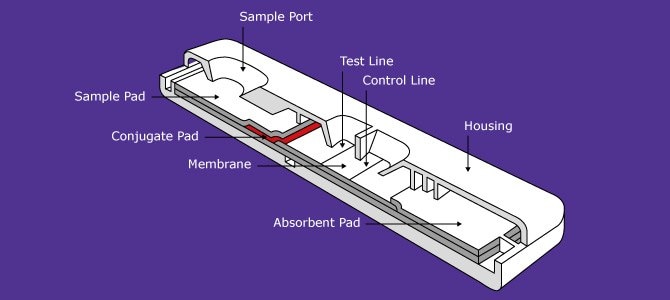
Figure 1.Lateral Flow Diagram
Taking a lateral flow test strip from the design stage through product development to final manufacturing is a process that employs principles from biology, chemistry, physics, and engineering. A test design that works well in the R&D lab is of little use if it is difficult to manufacture reliably in high yield. Similarly, even though a product may work well during in-house testing, if it fails to work reliably in the field, it is unlikely to be successful. Uncharacterized variations in a single material, reagent, or process can be sufficient to ruin consistency of performance.
With these considerations in mind, the purpose of this guide is to provide information on the key aspects of immunochromatographic test strip design, particularly the materials used and their integration with reagents and manufacturing processes. Understanding these principles will help to provide a framework for more rapid test development.
Getting Started
Product Description
Before starting test development, it is important to have a basic product design established. There are numerous product qualities that should be determined at the outset so that resources are not spent on a design that isn’t marketable. Necessarily, market information will be required to establish certain performance criteria. Key test parameters include: detection limits (sensitivity), required specificity, assay speed, detection format, detection system, test line format, results interpretation, sample matrices, housing design, packaging, labeling, stability requirements, and target cost. It is also critical to understand how much time is available for the test development program.
Antibodies and Other Capture Reagents
Antibodies used in immunochromatographic tests must have sufficient sensitivity, specificity, purity, and stability to accomplish the performance requirements of the finished product. Depending on the assay design, antibodies may be used as a capture reagent at the test line, as a conjugate on the detector particle, or both. As a manufacturing consideration, the antibodies should be available in sufficient supply to meet production forecasts. Purification and consistency of supply are also important. Since the antibodies may be bound to a membrane and detector particle, contaminating proteins will compete for binding sites. There is also the decision to be made as to whether polyclonal or monoclonal antibodies will be used. Minimally, the antibody preparation should be affinity-purified.
A key consideration in antibody selection is the chemical extremes to which the antibodies will be subjected during test strip manufacture and storage. Minimally, the antibody must remain reactive after being adsorbed to a solid surface, retain its structural integrity when completely dried, and then be instantly reactive when rehydrated by the sample. Antibody systems derived from other assays such as ELISA or western blots may not meet these requirements, necessitating screening for alternatives. Also, strategies used to stabilize antibodies in solution are often incompatible with immunochromatography tests.
While formation of an immunocomplex at the test line is most commonly used as the result indicator, it is theoretically possible to achieve a result using any ligand recognition system where a detector particle becomes bridged to a capture reagent on the membrane. In such systems the reagents employed, whether purified from a natural source or prepared as a synthetic construct, are subject to the same requirements for sensitivity and stability as antibodies. It is important to know how these reagents are prepared, what contaminants may be present in the ‘purified’ product, and what lot-to-lot variations can be anticipated. Purity should not be assumed, but rather demonstrated by the supplier using relevant analytical methods.
Detector Reagents
Various types of detector reagents can be used for the visualization of a signal. The most commonly used materials in commercially available tests are latex beads and colloidal gold particles. Other possibilities include enzyme conjugates, other colloidal metals, fluorescent particles, and magnetic particles. One of the most important features of the particles is that the population is monodisperse with consistency of size and spherical shape. When a test is run, the particles are required to move through the torturous pore structure of the membrane.
Smaller particles move faster than larger particles. Particle preparations with different size and shape distributions will move through the membrane differently. This can lead to differences in apparent sensitivity and specificity, even when all other components of the test are identical.
Methods for preparing various particles and conjugating antibodies to them can be found in the literature.
Commercial sources are also available. Since the conjugated detector particles are one of the key reagents in the finished test strip, methods for their preparation and handling should be fully validated. Similarly, relevant quality control methods need to be established. If they are purchased from a vendor, they should come with specifications on the attributes relevant to performance in lateral flow tests.
Licensing Agreements
There are many patents covering technologies, formats, reagents, and materials that may be of great value in the development of immunochromatographic test devices. Test developers may need to consider licensing one or more of these patents prior to the commercialization of final product. We do not provide legal counsel, nor can we assign rights for any of the patents that have been issued. A list of pertinent patents can be found in Table 1.
Some patents (e.g., US4855240, US4703017, US4376110 and EP0810436A1) are broadly based and may apply to many different test formats. Other patents are more restricted and may not apply based on reagent selection and test device formatting. Finally, there may be additional patents that pertain to immunochromatographic tests and immunodiagnostic assays in general. To comply with international trade and licensing agreements and to prevent possible legal problems after product launch, it is prudent for test developers and manufacturers to review the patent literature prior to commercialization.
Have a Manufacturing Plan in Each Phase of Development
Manufacturing schemes range from entirely manual to completely automated. For reproducibility, there are certain steps that require a high level of consistency.
Choose the Right Manufacturing Process
The choice of process depends primarily on the amount of product to be manufactured and the funds available to staff and equip the manufacturing facility.
- Manual – process materials in a batch mode.
- Automated – process materials in a continuous mode.
- Combination of manual and automated.
Key Elements for Manufacturing Consistency
Consider the following elements of the manufacturing process to ensure consistency from R&D through manufacturing.
- Consistent lamination of membranes, sample pads, conjugate pads, and absorbent pads onto a support backing.
- Precision cutting of sheets or rolls into strips of defined length and width.
- Control for consistency of reagent application onto membranes, sample pads, conjugate pads, and other porous media.
- Placement of test strips in plastic housings.
Minimize Waste, Maximize Yield
And finally, when designing the assay and planning for progression to a manufactured product, use the following steps to minimize waste and maximize yield.
- Match lot sizes of materials and reagents during planning.
- Continuously train personnel to ensure proper execution of manufacturing processes.
- Continuously conduct in-process inspections to ensure sub-assemblies are being manufactured to specification.
- Ensure that materials chosen are compatible with manufacturing machinery so they perform predictably in the finished device.
To continue reading please sign in or create an account.
Don't Have An Account?