132039
1,2,4-Triacetoxybenzene
97%
Synonym(s):
1,2,4-Phenenyl triacetate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3CO2)3C6H3
CAS Number:
Molecular Weight:
252.22
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
assay
97%
form
solid
mp
98-100 °C (lit.)
SMILES string
CC(=O)Oc1ccc(OC(C)=O)c(OC(C)=O)c1
InChI
1S/C12H12O6/c1-7(13)16-10-4-5-11(17-8(2)14)12(6-10)18-9(3)15/h4-6H,1-3H3
InChI key
AESFGSJWSUZRGW-UHFFFAOYSA-N
Related Categories
Application
1,2,4-Triacetoxybenzene can be used as a starting material:
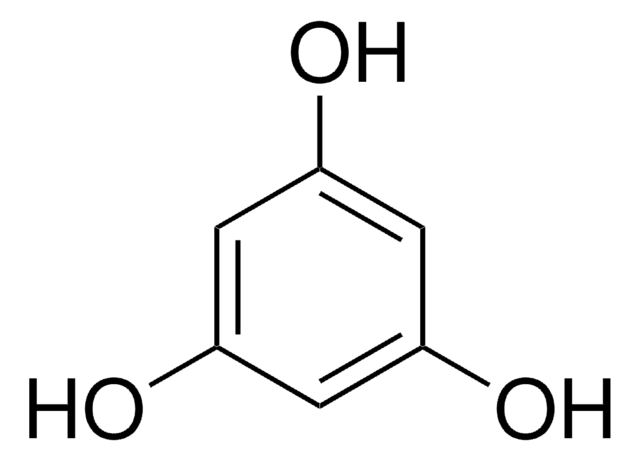
- To prepare aminated hydroxynaphthazarins, echinamines A and B.
- To synthesize 6,7-dihydroxychromenones, which are further used to prepare various crown ethers.
- In the total synthesis of natural product santalin Y.
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nataly D Pokhilo et al.
Journal of natural products, 69(8), 1125-1129 (2006-08-29)
The first total synthesis of two marine aminated hydroxynaphthazarins, echinamines A (3-amino-7-ethyl-2,5,6,8-tetrahydroxy-1,4-naphthoquinone) and B (2-amino-7-ethyl-3,5,6,8-tetrahydroxy-1,4-naphthoquinone), produced by the sea urchin Scaphechinus mirabilis is described. This was achieved from 1,2,4-triacetoxybenzene (13) through a sequence involving double Fries rearrangement of 13, reduction
Synthesis of Echinamines A and B, the First Aminated Hydroxynaphthazarins Produced by the Sea Urchin Scaphechinus m irabilis and Its Analogues
Pokhilo ND, et al.
Journal of Natural Products, 69, 1125-1129 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service